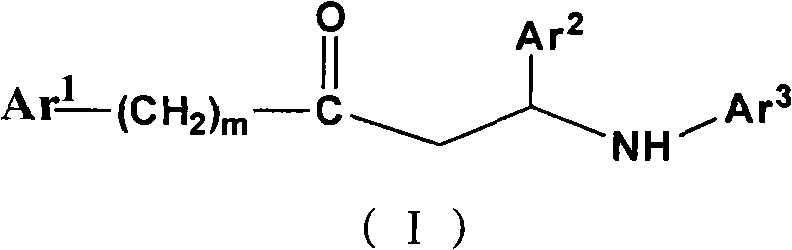
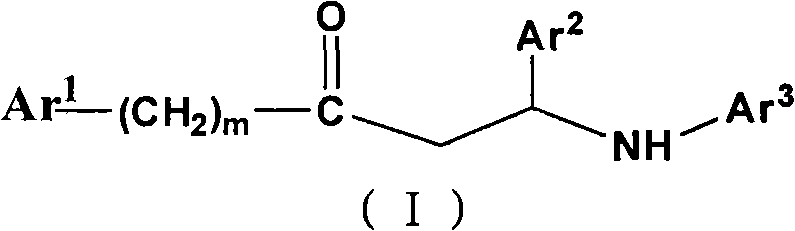
Beta-amino ketones compound with anti-diabetic activity
An anti-diabetic, amino ketone technology, applied in the direction of organic active ingredients, active ingredients of heterocyclic compounds, organic chemistry, etc., can solve problems such as toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0473] Example 1, Ydcm230: 4-(3-(4-chlorophenyl)-3-oxo-1-m-tolylamino)benzenesulfonamide
[0474] 12.00 grams (100 mmol) of 3-methylbenzaldehyde, 17.22 grams (100 mmol) of p-aminobenzenesulfonamide, and 150 ml of absolute ethanol were added to the reaction flask, and after stirring at room temperature for 10 minutes, 15.25 grams (100 mmol) of 4-chloroacetophenone was added. ) and a catalytic amount of concentrated hydrochloric acid, and then stirred and reacted at 30° C. for 44 hours. After the reaction, the reaction solution was cooled overnight, and the precipitated solid was filtered with suction and washed with absolute ethanol. The resulting solid was suspended in 160ml of 95% ethanol, stirred at room temperature for 2 hours, and washed with 10% K 2 CO 3 Neutralize the solution to alkaline, filter with suction, wash the filter cake with a small amount of absolute ethanol, and recrystallize the crude product from a mixed solvent of ethanol / water (volume ratio 1:1) to obt...
Embodiment 2
[0475] Example 2, Ydcm 231: 4-(3-(4-chlorophenyl)-3-oxo-1-p-chlorophenylpropylamino)benzenesulfonamide
[0476] Add 14.05 grams (100 mmol) of 4-chlorobenzaldehyde, 17.22 grams (100 mmol) of p-aminobenzenesulfonamide, and 150 ml of absolute ethanol into the reaction flask, stir at room temperature for 10 minutes, then add 15.25 grams (100 mmol) of 4-chloroacetophenone and a catalytic amount of concentrated hydrochloric acid, then stirred at 30°C for 31 hours. After the reaction, the reaction solution was cooled overnight, and the precipitated solid was filtered with suction and washed with absolute ethanol. The resulting solid was suspended in 160ml of 95% ethanol, stirred at room temperature for 2 hours, and washed with 10% K2 CO 3 Neutralize the solution to alkaline, filter with suction, wash the filter cake with a small amount of absolute ethanol, and recrystallize the crude product from a mixed solvent of ethanol / water (volume ratio 1:1) to obtain 27.61 g of crystals, with...
Embodiment 3
[0477] Example 3, Ydcm 232: 4-(3-(4-chlorophenyl)-3-oxo-1-phenylpropylamino)benzenesulfonamide
[0478] Add 10.62 grams (100 mmol) of benzaldehyde, 17.22 grams (100 mmol) of p-aminobenzenesulfonamide, and 150 ml of absolute ethanol into the reaction flask. After stirring at room temperature for 10 minutes, add 15.25 grams (100 mmol) of 4-chloroacetophenone and a catalytic amount of Concentrated hydrochloric acid, and then stirred at 30°C for 72 hours. After the reaction, the reaction solution was cooled overnight, and the precipitated solid was filtered with suction and washed with absolute ethanol. The resulting solid was suspended in 160ml of 95% ethanol, stirred at room temperature for 2 hours, and washed with 10% K 2 CO 3 Neutralize the solution to alkaline, filter with suction, wash the filter cake with a small amount of absolute ethanol, and recrystallize the crude product from a mixed solvent of ethanol / water (volume ratio 1:1) to obtain 27.61 g of crystals, with a yi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com