Insulin oral nano-preparation and preparation method thereof
A nano-preparation and insulin technology, applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problems of being easily damaged by the gastrointestinal environment, poor patient compliance, and short biological half-life, etc. problems, achieve good development prospects, good biocompatibility, and prolong the effect of validity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
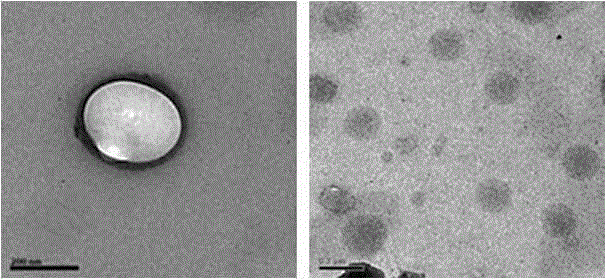
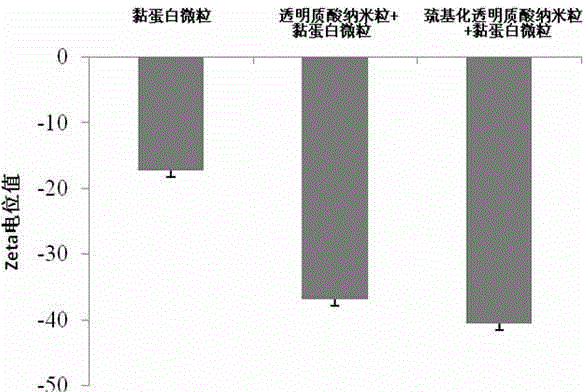
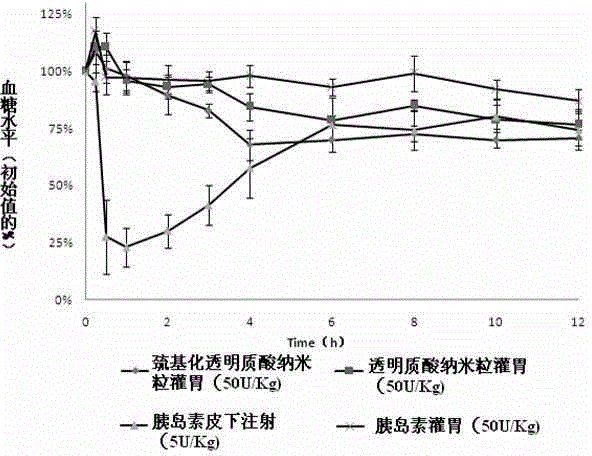
[0023] (1) Preparation of nanosuspension: Weigh thiolated hyaluronic acid and insulin and dissolve them in the water phase, wherein the mass ratio of thiolated hyaluronic acid to insulin is 0.5:1-10:1; add surface The active agent can be Tween 80, soybean lecithin, egg yolk lecithin, macrogolglyceride oleate, macrogolglyceride caprylate, macrogol 40 hydrogenated castor oil, polyoxyethylene 35 castor oil , one or more mixtures of polyethylene glycol 15 hydroxystearate, the dosage is 0.1%-10% (w / v); the organic phase is dichloromethane, acetone, cyclohexane, n-hexane, One or more mixtures of diethyl ether; surfactants added to the organic phase are macrogol glyceride oleate, Span 80, macrogol glyceride caprate caprylate, macrogol 40 hydrogenated castor oil , polyoxyethylene 35 castor oil, polyethylene glycol 15 hydroxystearate or a mixture of more than one, the dosage is 0.1%-10% (w / v); the volume ratio of the aqueous phase to the organic phase is 5 :1-1:20; the ultrasonic powe...
Embodiment 1
[0025] Example 1 Preparation of Oral Nano-Preparation of Insulin
[0026]
[0027] Preparation process: Dissolve mercapto-hyaluronic acid and insulin in distilled water, add Tween 80, mix evenly as the water phase, slowly add the water phase into acetone containing polyoxyethylene 35 castor oil under magnetic stirring, and ultrasonic probe 300W , 5min, to obtain W / O type colostrum; remove acetone by rotary evaporation at 40°C, add distilled water to 200ml, and then ultrasonically probe at 300W, 5min, to obtain insulin nanoparticle suspension; add lactose and mannose to insulin nanoparticle suspension Alcohol is used as a lyoprotectant, pre-frozen at -70°C for 8 hours, and freeze-dried for 48 hours.
Embodiment 2
[0028] Example 2 Preparation of Oral Nano-Preparation of Insulin
[0029]
[0030] Preparation process: Dissolve thiolated hyaluronic acid and insulin in distilled water, add Tween 80, mix evenly as the water phase, slowly add the water phase into dichloromethane containing soybean lecithin under magnetic stirring, ultrasonic probe at 250W for 3min , to obtain W / O type colostrum; remove acetone by rotary evaporation at 40°C, add distilled water to 200ml, and then ultrasonically probe at 250W for 3min to obtain insulin nanoparticle suspension; add glucose and mannitol to the insulin nanoparticle suspension as The lyoprotectant was pre-frozen at -70°C for 8 hours, and then freeze-dried for 48 hours.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com