Chitosan nanoparticle for improving absorption of orally delivered insulin by colon and preparation method of chitosan nanoparticle
A technology of chitosan nanoparticles and insulin, which is applied in the directions of non-active ingredients medical preparations, medical preparations containing active ingredients, pharmaceutical formulas, etc. The degree of utilization needs to be improved to achieve the effect of protective activity, good physical and chemical stability, and increased interaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
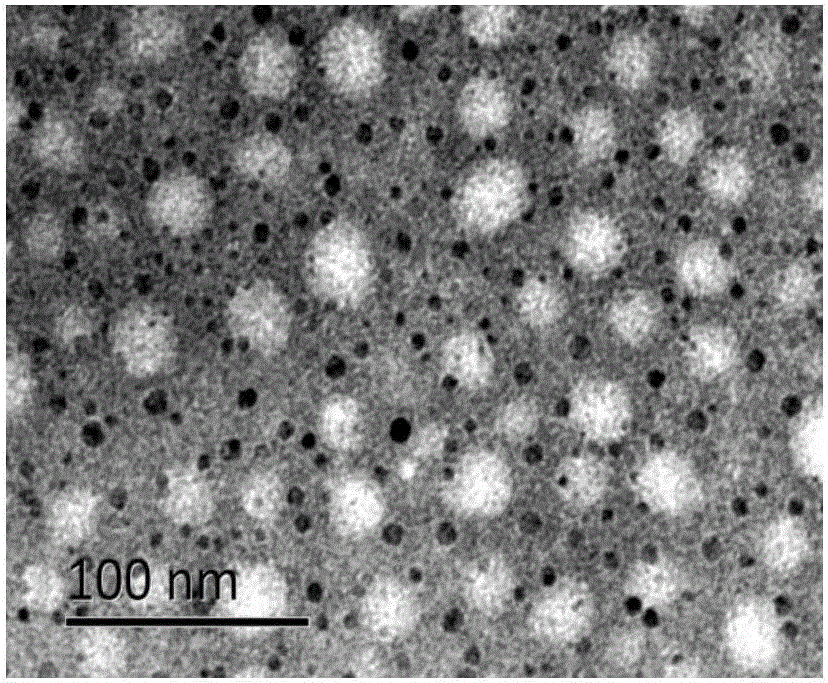
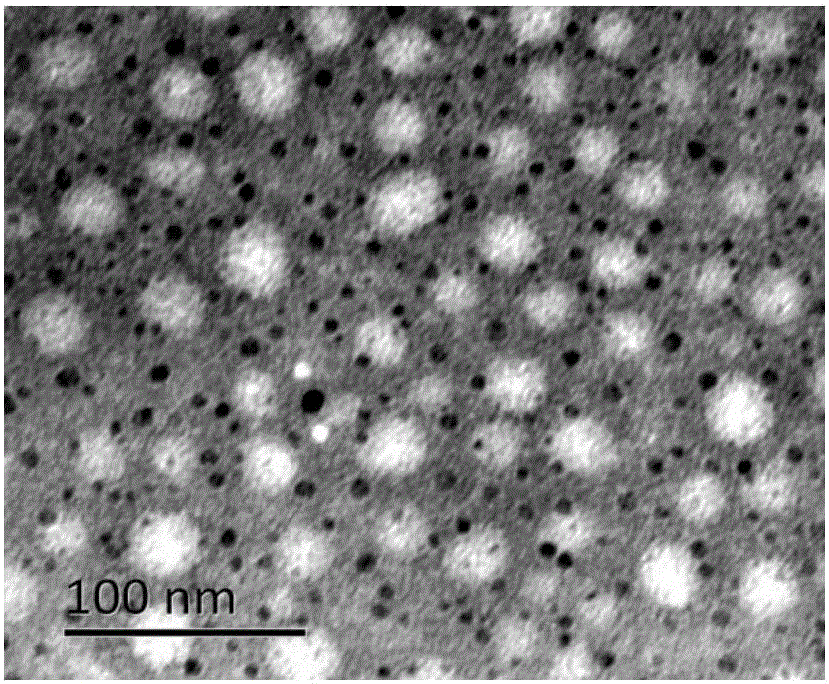
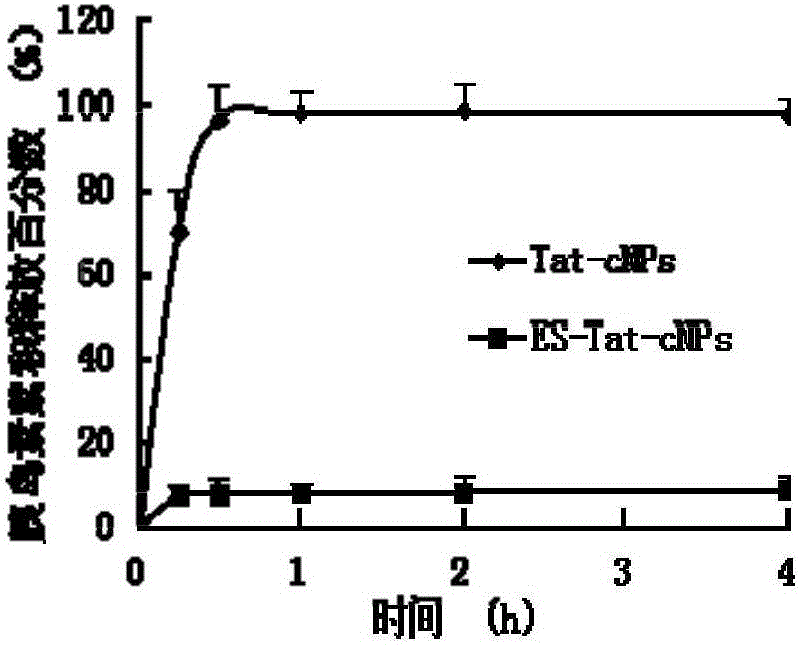
Image
Examples
Embodiment 1
[0076] Weigh 8 mg of chitosan powder with a molecular weight of 100,000 Daltons and a degree of deacetylation of 95%, dissolve it in 4 ml of 0.2% acetic acid solution, adjust the pH value to 5.5 with 1 mol / L sodium hydroxide solution, and call it Solution A. Weigh 2 mg of Tat (amino acid sequence GRKKRRQRRRP), dissolve it in solution A and call it solution B, and stir solution B on a magnetic stirrer at room temperature. Weigh 4.2 mg of insulin, dissolve it in 2 mL of 0.1 mol / L hydrochloric acid solution, adjust the pH value to 8.0 with 1 mol / L sodium hydroxide solution, and call it solution C. Solution C was slowly added to solution B under stirring at room temperature, and stirring was continued for 1 hour to form solution D. Weigh 2.3 mg of sodium tripolyphosphate and dissolve it in 2 mL of distilled water and call it solution E. Slowly add solution E to solution D while stirring at room temperature, and continue stirring for 0.5 hours to form nanoparticle suspension F. W...
Embodiment 2
[0078]Weigh 8 mg of chitosan powder with a molecular weight of 50,000 Daltons and a degree of deacetylation of 85%, dissolve it in 4 ml of 0.2% acetic acid solution, adjust the pH value to 6.0 with 1 mol / L sodium hydroxide solution, and call it Solution A. Weigh 2.3 mg of Tat (amino acid sequence GRKKRRQRRRP), dissolve it in solution A and call it solution B, and stir solution B on a magnetic stirrer at room temperature. Weigh 4 mg of insulin, dissolve it in 2 mL of 0.1 mol / L hydrochloric acid solution, adjust the pH value to 8.0 with 1 mol / L sodium hydroxide solution, and call it solution C. Solution C was slowly added to solution B under stirring at room temperature, and stirring was continued for 1 hour to form solution D. Weigh 2 mg of sodium tripolyphosphate and dissolve it in 2 mL of distilled water and call it solution E. Slowly add solution E to solution D while stirring at room temperature, and continue stirring for 0.5 hours to form nanoparticle suspension F. Weigh...
Embodiment 3
[0080] Weigh 8 mg of chitosan powder with a molecular weight of 100,000 Daltons and a degree of deacetylation of 85%, dissolve it in 4 ml of 0.2% acetic acid solution, adjust the pH value to 5.5 with 1 mol / L sodium hydroxide solution, and call it Solution A. Weigh 2 mg of Tat (amino acid sequence GRKKRRQRRRP), dissolve it in solution A and call it solution B, and stir solution B on a magnetic stirrer at room temperature. Weigh 4 mg of insulin, dissolve it in 2 mL of 0.1 mol / L hydrochloric acid solution, adjust the pH value to 8.0 with 1 mol / L sodium hydroxide solution, and call it solution C. Solution C was slowly added to solution B under stirring at room temperature, and stirring was continued for 1 hour to form solution D. Weigh 2.3 mg of sodium tripolyphosphate and dissolve it in 2 mL of distilled water and call it solution E. Slowly add solution E to solution D while stirring at room temperature, and continue stirring for 0.5 hours to form nanoparticle suspension F. Wei...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com