Application of compound in preparation of drug for treating type 2 diabetic cardiomyopathy
A technology for type 2 diabetes and a compound is applied in the application field of the compound in the preparation of a drug for the treatment of type 2 diabetic cardiomyopathy, and can solve the problems of lack of specific drugs, therapeutic targets, side effects and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
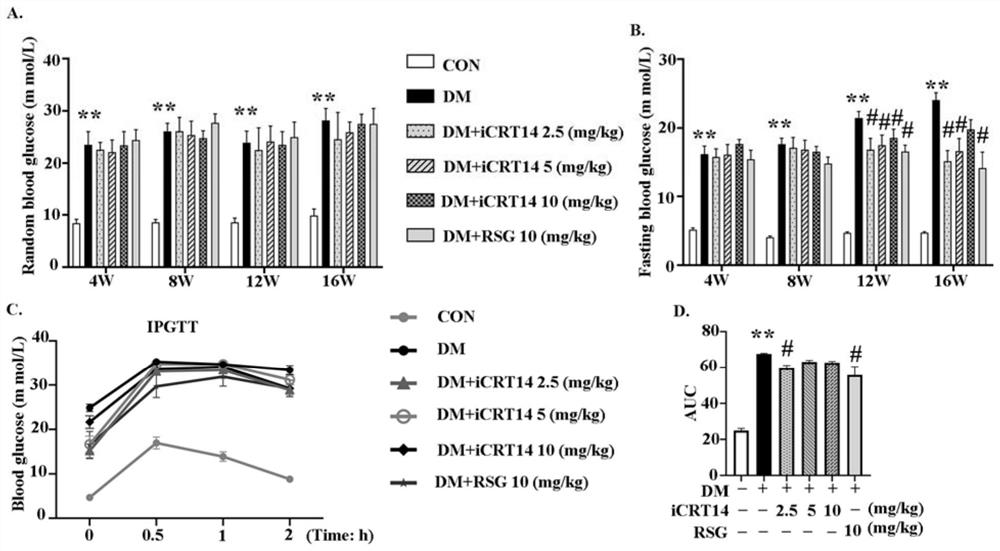
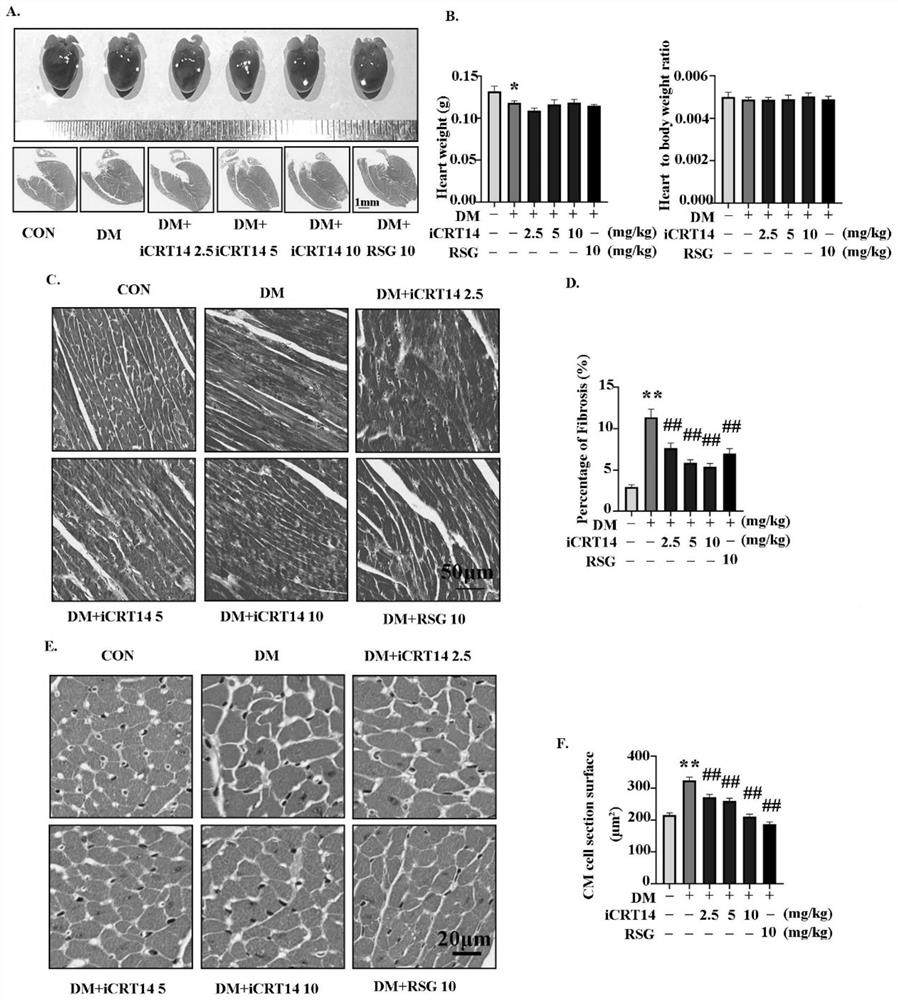
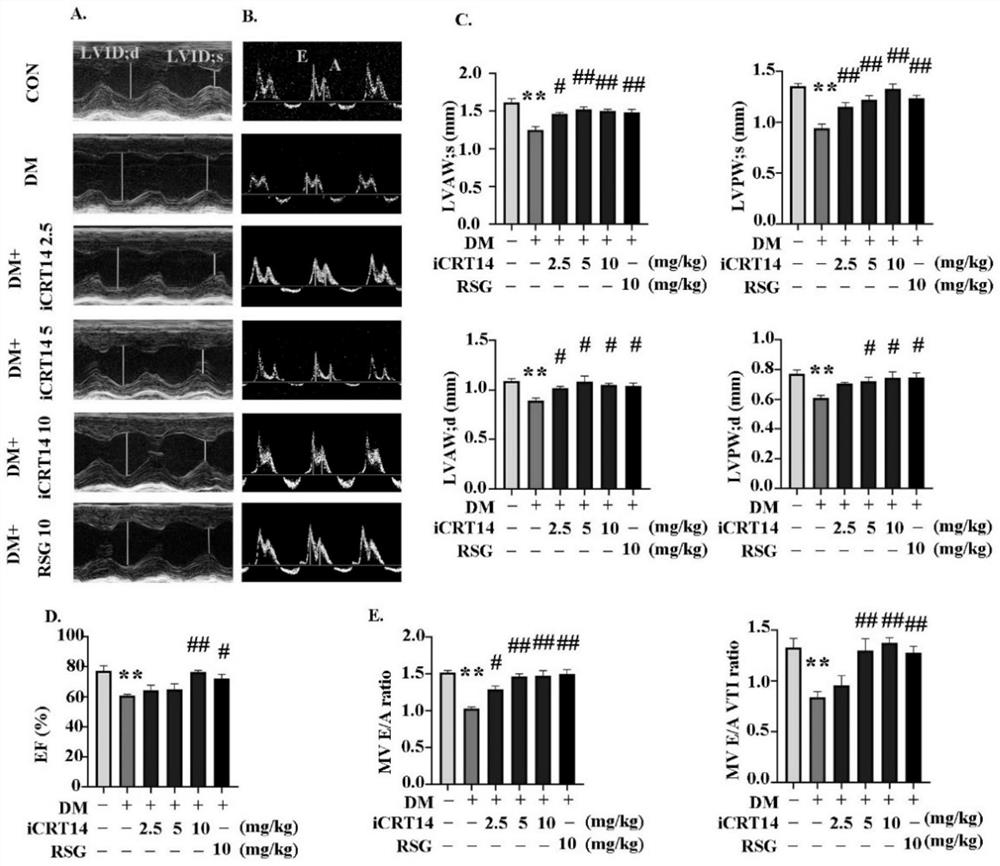
[0028] Example 1 Effect test of the compound (iCRT14) of the present invention in the treatment of type 2 diabetic cardiomyopathy 1. Experimental method
[0029] 1. Construction of animal models of type 2 diabetes
[0030]Select 3-4 week-old C57BL / 6J male mice as the test subjects, after 2 weeks of high-fat diet, intraperitoneally inject STZ (85mg / kg), inject once every other day, fast for 12h before intraperitoneal injection, and avoid light The injection was completed within 30 minutes, and the animal model of type 2 diabetes was constructed, followed by high-fat feeding. The random blood glucose of mice ≥ 16.7mmol / L can be used as a model group for experiments. Mice of the same age and sex are the normal control group (Control group, CON) , mice in the normal control group had normal diet and free access to water.
[0031] 2. Main solution configuration
[0032] (1) iCRT14 suspension injection: Weigh the injection dose, add 20% PEG300 and 80% PBS solution, and mix on a vo...
Embodiment 2
[0099] Embodiment 2 Compound (iCRT14) described in the present invention pharmacokinetic detection test in mice
[0100] At present, the data on the pharmacokinetics of iCRT14 is extremely limited. We established and verified a sensitive and highly selective method to detect the concentration of iCRT14 in mouse plasma for the study of iCRT14 pharmacokinetics.
[0101] In this example, the analyte was protein precipitated with methanol. The LC-ESIMS / MS method uses an Agilent Eclipse Plus C18 column (2.1 mm×50 mm, 1.8 μm), and the mobile phase is methanol-water (containing 0.1% formic acid). The linear range is 2.5~2000ng / mL. This method was used to study the pharmacokinetic characteristics of iCRT14 after intravenous and intraperitoneal administration in mice.
[0102] experimental method:
[0103] 1. 24 C57BL / 6J mice aged 7-8 weeks were randomly divided into two groups: intravenous injection group (Iv) and intraperitoneal injection group (Ip). The injection solution is dis...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com