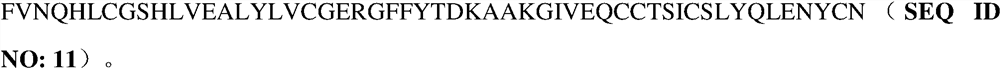Structure of a novel proinsulin aspart and method for preparing insulin aspart
A technology of insulin aspart and sequence, which is applied in the direction of insulin, chemical instruments and methods, botany equipment and methods, etc., can solve the problems of inefficiency, time-consuming, expensive, etc., achieve simple purification process, avoid the problem of impurities, and remove and purify The effect of steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0099] Example 1: Construction of an Escherichia coli clone expressing formula I novel proinsulin.
[0100] One is designed for expressing the proinsulin protein sequence as shown in formula I in escherichia coli. The N-terminal leading amino acid sequence can enhance expression, protect proinsulin aspart, and prevent degradation by Escherichia coli. The preferred leader amino acid sequence is MATHAVSVLKGDGPVQGIINFEQHESNGPVKVWGSIHGLTEGLHGFHVHEFGDNTAGSTSAGP (SEQ ID NO: 1);
[0101] The C-terminal of the leader amino acid sequence is connected to the B chain of insulin aspart through arginine or lysine residues, and the leader peptide is removed by trypsin cleavage. The C-peptide of proinsulin is shortened to an arginine or lysine residue, and the precursor sequence of the novel proinsulin is:
[0102]
[0103] Full sequence of proinsulin proaspart band leader peptide MATHAVSVLKGDGPVQGIINFEQHESNGPVKVWGSIHGLTEGLHGFHVHEFGDNTAGSTSAGPRFVNQHLCGSHLVEALYLVCGERGFFYTDKTRGIVEQCCTSICS...
example 2
[0105] Example 2: Expression of proinsulin proaspart fusion protein
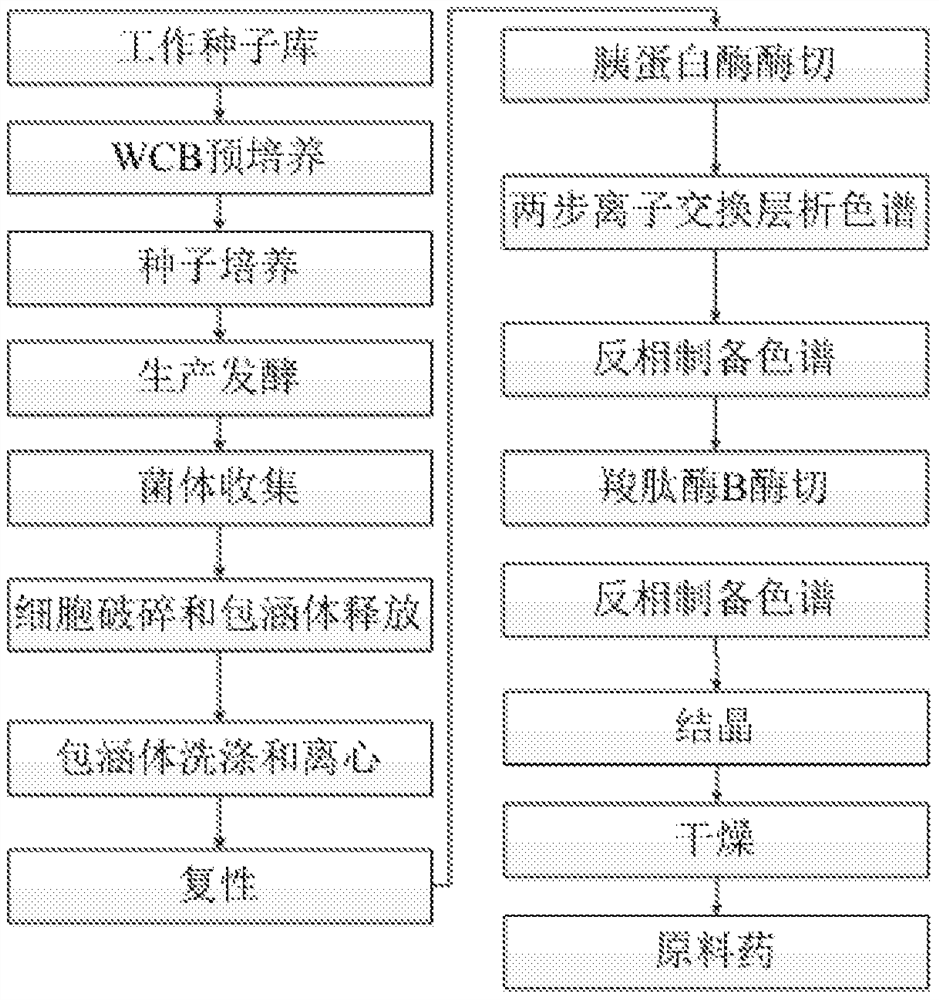
[0106] The WCB obtained in Example 1 was cultured at 37° C. for 8 hours in LB medium (containing 1.5% w / v yeast powder and 0.5% w / v sodium chloride) to obtain a cell seed solution. The seed solution was inoculated into BFM medium (containing 0.6% w / v diammonium hydrogen phosphate, 0.4% w / v ammonium chloride, 1.35% w / w potassium dihydrogen phosphate, 0.139% w / w magnesium sulfate 7 water, 0.28 %w / w citric acid monohydrate, 0.8%w / w glucose, 0.3%w / w yeast powder and 1%mL / L trace element solution) were further cultivated for 8h to obtain the secondary seed liquid, and then inoculated according to the volume ratio of 1:10 to the fermenter. Cultivate for about 16 hours until the OD600 of the fermentation broth is about 150, and then add IPTG at a final concentration of 1 mM to the fermenter to induce the expression of proinsulin proaspart. Induced for 12 hours, the fermentation process was completed. Bacteria we...
example 3
[0109] Example 3: Refolding of proinsulin aspart
[0110] The inclusion body solution was filtered with a 1 μm PP filter element, the temperature was controlled at 20° C., the pH was adjusted to 11.0, and then refolded for 24-48 hours to obtain refolded proinsulin aspart.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com