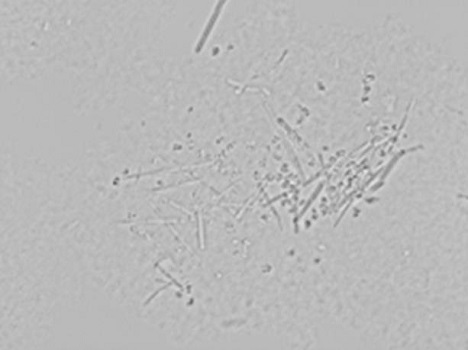
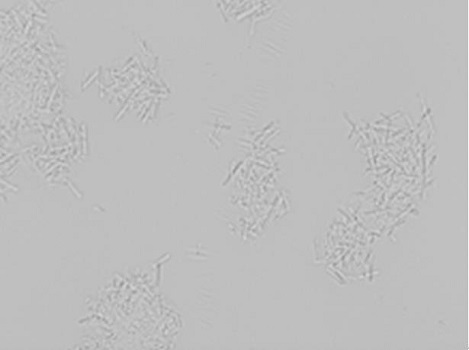
Insulin aspart crystallization process
A technology of insulin aspart and crystallization, which is applied in the field of biomedicine, can solve the problems affecting the quality of insulin products and the difficulty of effectively controlling the content of crystalline insulin, and achieve high production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Insulin aspart crystals were prepared as follows:
[0037] (1) Weigh insulin aspart, glycerin, phenol, m-cresol, sodium chloride, disodium hydrogen phosphate dihydrate and zinc chloride;
[0038] (2) Fully dissolve about 2.45 g of the insulin aspart with an appropriate amount of 0.1 mol / L dilute hydrochloric acid to prepare an insulin aspart solution; dissolve 9.6 g of glycerin, 900 mg of phenol, and m-cresol 1032mg, sodium chloride 526.2mg, disodium hydrogen phosphate dihydrate 750mg; the amount of zinc chloride added to zinc is 13.72mg to obtain adjuvant solution; the insulin aspart solution is mixed with adjuvant solution, and ultrapure water Dilute to 600ml, and adjust the pH value to 7.1-7.44 with 0.5M sodium hydroxide solution;
[0039] (3) Take about 320mg of protamine sulfate, after swelling, add 1.6g of glycerin, 150mg of phenol, 172mg of m-cresol, 87.7mg of sodium chloride, 125mg of disodium hydrogen phosphate dihydrate, and use ultrapure water to prepare pro...
Embodiment 2
[0043] Insulin aspart crystals were prepared as follows:
[0044] (1) Weigh insulin aspart, glycerin, phenol, m-cresol, sodium chloride, disodium hydrogen phosphate dihydrate and zinc chloride;
[0045] (2) Fully dissolve about 2.45 g of the insulin aspart with an appropriate amount of 0.1 mol / L dilute hydrochloric acid to prepare an insulin aspart solution; dissolve 11.2 g of glycerin, 1050 mg of phenol, and m-cresol 1204mg, sodium chloride 613.9mg, disodium hydrogen phosphate dihydrate 875mg; the amount of zinc chloride added to zinc is 13.72mg to obtain adjuvant solution; the insulin aspart solution is mixed with adjuvant solution, and ultrapure water Dilute to 600ml, and adjust the pH value to 7.1-7.44 with 0.5M sodium hydroxide solution;
[0046] (3) Take about 320mg of protamine sulfate, after swelling, use ultrapure water to prepare 100ml of protamine sulfate solution;
[0047] (4) Mix the mixed solution obtained in step (2) with the protamine sulfate solution obtaine...
Embodiment 3
[0050] Insulin aspart crystals were prepared as follows:
[0051] (1) Weigh insulin aspart, glycerin, phenol, m-cresol, sodium chloride, disodium hydrogen phosphate dihydrate and zinc chloride;
[0052] (2) Fully dissolve about 2.45 g of the insulin aspart with an appropriate amount of 0.1 mol / L dilute hydrochloric acid to prepare an insulin aspart solution; dissolve 11.2 g of glycerin, 1050 mg of phenol, and m-cresol 1204mg, sodium chloride 613.9mg, disodium hydrogen phosphate dihydrate 1250mg; the amount of zinc chloride added to zinc is 13.72mg to obtain adjuvant solution; the insulin aspart solution is mixed with adjuvant solution, and ultrapure water Dilute to 600ml, and adjust the pH value to 7.1-7.44 with 0.5M sodium hydroxide solution;
[0053] (3) Take about 320mg of protamine sulfate, after swelling, use ultrapure water to prepare 100ml of protamine sulfate solution;
[0054] (4) Mix the mixed solution obtained in step (2) with the protamine sulfate solution obtain...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com