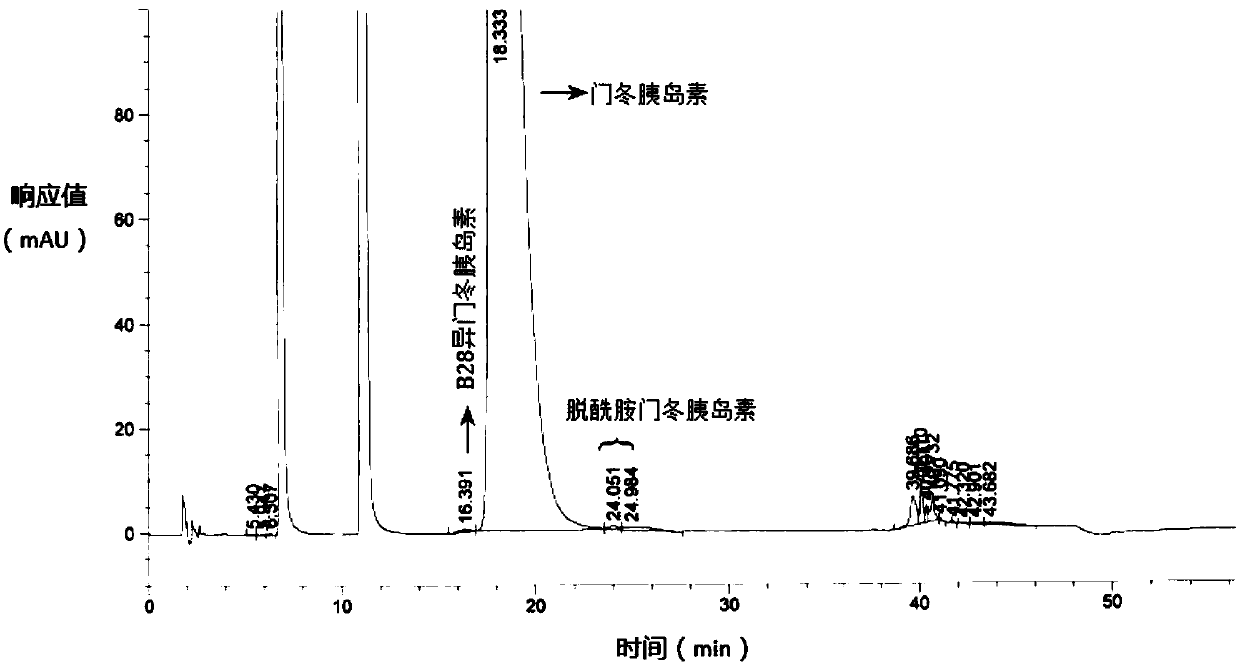
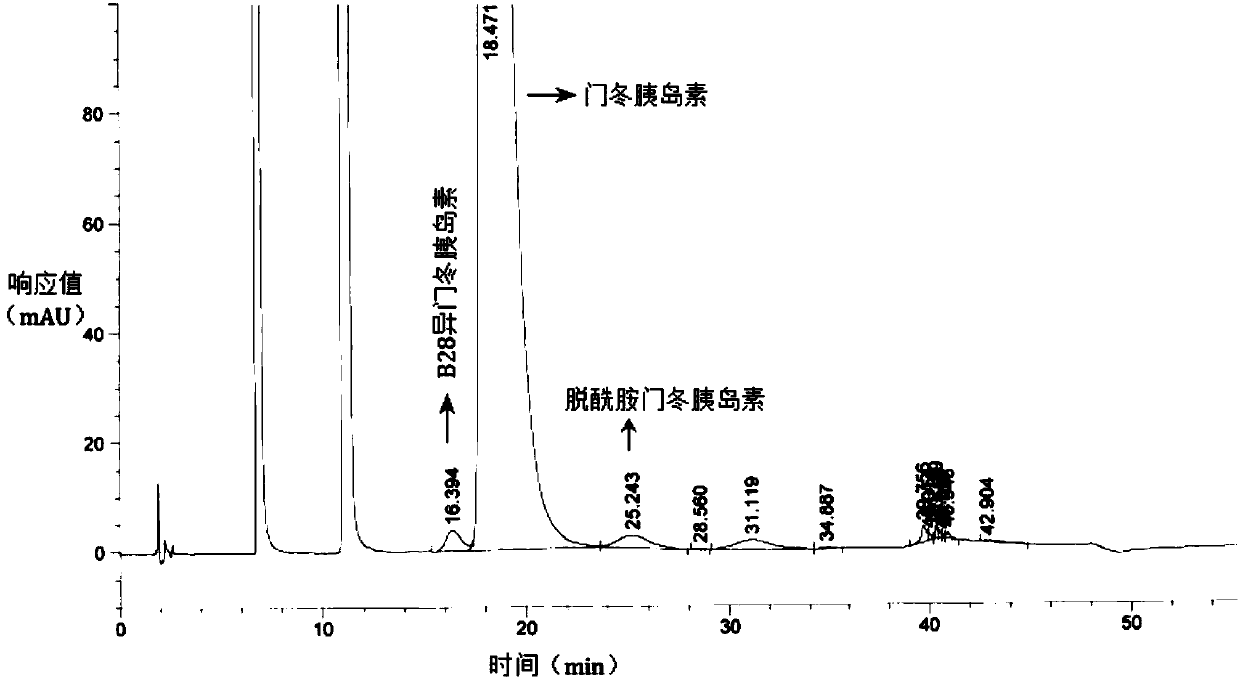
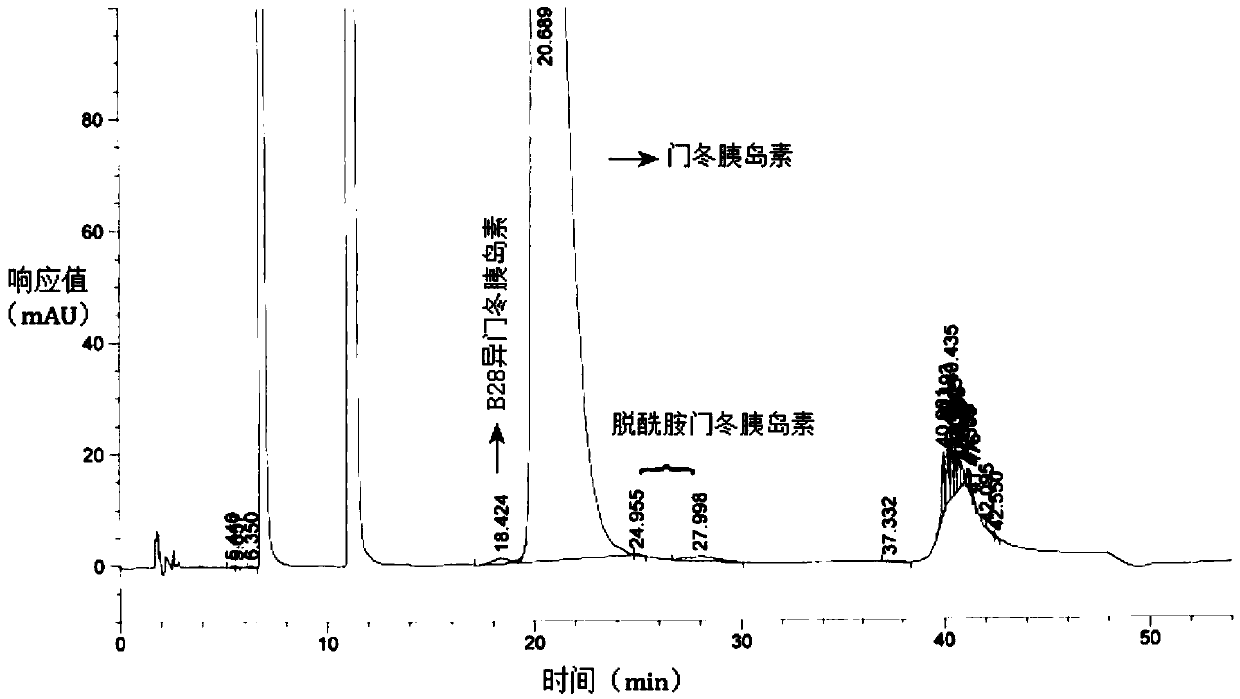
Preparation method of insulin aspart injection
A technology for preparing insulin aspart and drugs, which is applied to medical preparations with non-active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, which can solve problems such as time-consuming, limited production efficiency, and labor, and achieve increased stability. , Simplify the process steps and increase the safety effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] 1. Prescription
[0047] insulin aspart
35g
glycerin
160g
15g
m-cresol
17.2g
0.196g
5.8g
10g
[0048] 2. Solution preparation:
[0049] a): Weigh the disodium hydrogen phosphate of the prescribed amount, dissolve it with 4L of water for injection and add it to the liquid mixing tank;
[0050] b): Weigh the glycerin, phenol, m-cresol, sodium chloride, and zinc chloride of the prescribed amount, dissolve them in 1L of water for injection, and then add them to the liquid mixing tank in sequence;
[0051] c): the solution is stirred and mixed evenly;
[0052] d): Add the prescribed amount of insulin aspart powder into the liquid preparation tank while stirring, and mix and dissolve;
[0053] e): Supplement water for injection to 10L;
[0054] f): The medicinal solution is sterilized by filtering through a 0.22 μm filter membrane.
Embodiment 2
[0056] 1. Prescription
[0057] insulin aspart
35g
glycerin
160g
phenol
15g
m-cresol
17.2g
Zinc chloride
0.196g
Sodium chloride
5.8g
10g
[0058] 2. Solution preparation:
[0059] a): Weigh the disodium hydrogen phosphate of the prescribed amount, dissolve it with 4L of water for injection and add it to the liquid mixing tank;
[0060] b): Add the prescribed amount of insulin aspart powder into the liquid preparation tank while stirring, and mix and dissolve;
[0061] c): Weighing glycerin, phenol, m-cresol, sodium chloride, and zinc chloride of the prescribed amount are dissolved in 1L of water for injection respectively, and then added to the liquid mixing tank in sequence;
[0062] d): the solution is stirred and mixed evenly;
[0063] e): Supplement water for injection to 10L;
[0064] f): The medicinal solution is sterilized by filtering through a 0.22 μm filter membrane. ...
Embodiment 3
[0066] 1. Prescription
[0067] insulin aspart
35g
glycerin
160g
phenol
30g
Zinc chloride
0.196g
Sodium chloride
5.8g
10g
[0068] 2. Solution preparation:
[0069] a): Weigh the disodium hydrogen phosphate of the prescribed amount, dissolve it with 4L of water for injection and add it to the liquid mixing tank;
[0070] b): add the insulin aspart powder of the prescribed amount while stirring in the liquid preparation tank, mix and dissolve;
[0071] c): Glycerin, phenol, sodium chloride, and zinc chloride of the prescription amount were weighed and dissolved in 1L of water for injection respectively, and then added to the liquid mixing tank in sequence;
[0072] d): the solution is stirred and mixed evenly;
[0073] e): Supplement water for injection to 10L;
[0074] f): The medicinal solution is sterilized by filtering through a 0.22 μm filter membrane.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com