Preparation method of insulin aspart
A technology for insulin aspart and preparations, which is applied in the biological field and can solve problems such as weak expression ability, complicated process, and increased drug production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0178] Construction and expression of embodiment 1 insulin aspart expression strain
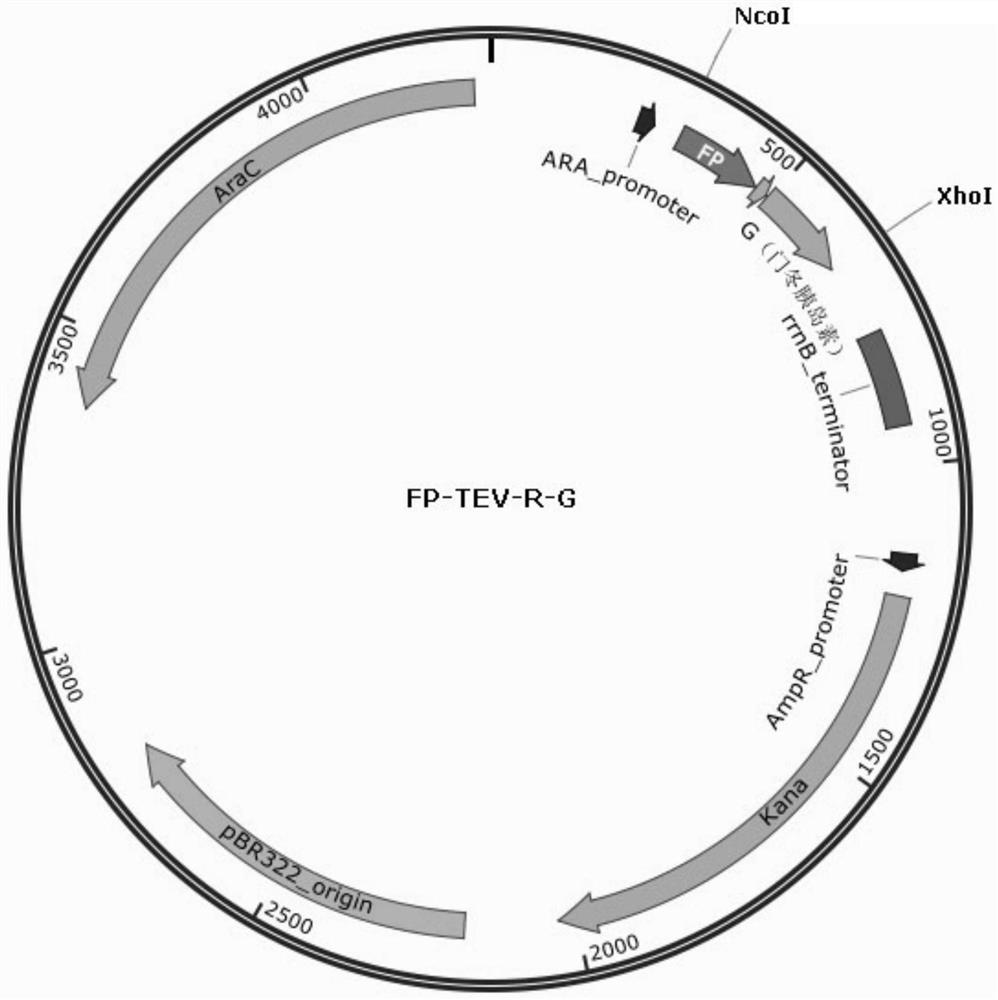
[0179] The construction of the insulin aspart expression plasmid refers to the description in the examples in the patent application number 201910210102.9. The DNA fragment containing the fusion protein FP-TEV-R-G was cloned into the NcoI-XhoI site downstream of the araBAD promoter of the expression vector plasmid pBAD / His A (purchased from NTCC, kanamycin resistance) to obtain the plasmid pBAD- FP-TEV-R-G. Plasmid map such as figure 1 shown.
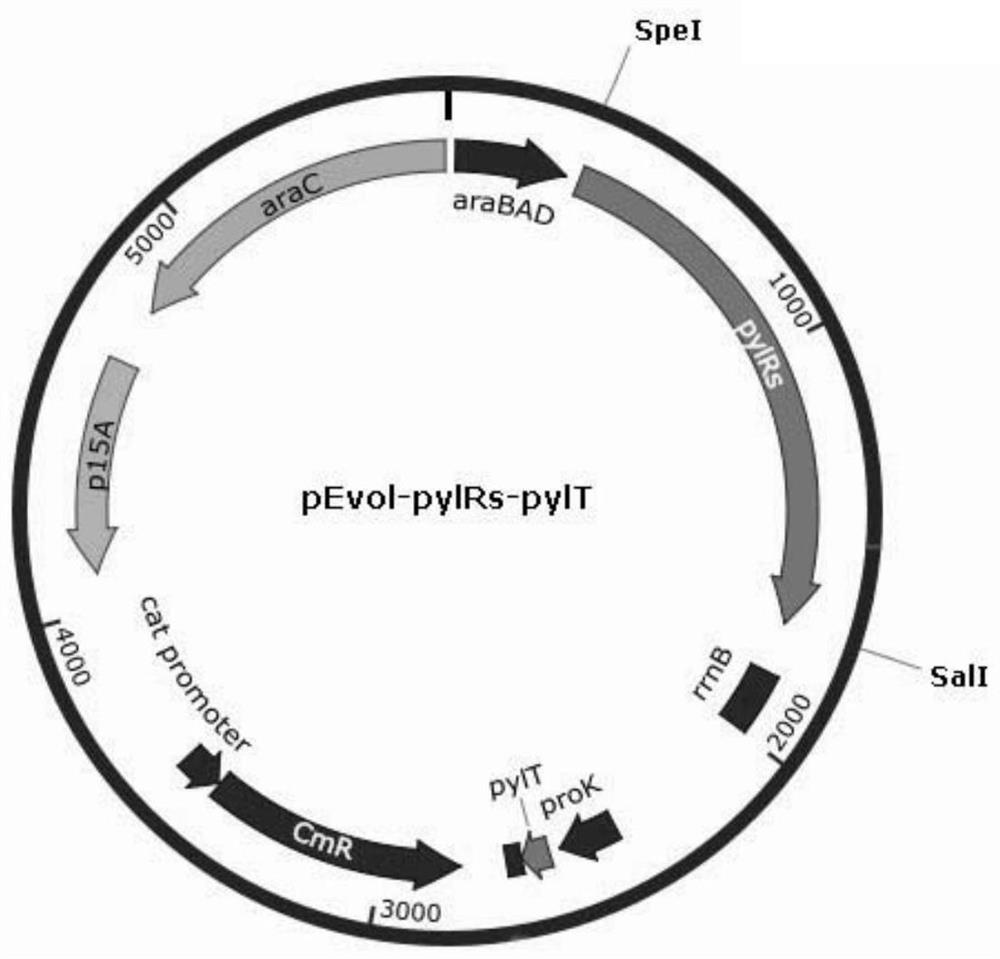
[0180] Then the DNA sequence of pylRs was cloned into the SpeI-SalI site downstream of the araBAD promoter of the expression vector plasmid pEvol-pBpF (purchased from NTCC Company, chloramphenicol resistance), and at the same time, the pylRs was inserted downstream of the proK promoter by PCR. DNA sequence of tRNA (pylTcua) of aminoacyl-tRNA synthetase. This plasmid was named pEvol-pylRs-pylT. Plasmid map such as figure 2 shown.
[0181] The c...
Embodiment 2
[0193] Example 2 Dissolution and renaturation of inclusion bodies
[0194]Add 8 mol / L urea solution to the obtained inclusion bodies, adjust the pH to 9.0-10.0 with sodium hydroxide, stir at room temperature for 1-3 hours, control the protein concentration to 10-20 mg / mL, and add β-mercaptoethanol to a final concentration of 15-20mmol / L, continue stirring for 0.5-1.0h.
[0195] Add the inclusion body solution dropwise to the renaturation buffer, dilute 5-10 times for renaturation, maintain the pH of the renaturation solution at 9.0-10.0, and stir for 10-20 hours for renaturation.
[0196] After 20 hours of renaturation, the fusion content of insulin aspart with correct renaturation and folding was detected by HPLC, and the renaturation rate was over 75%.
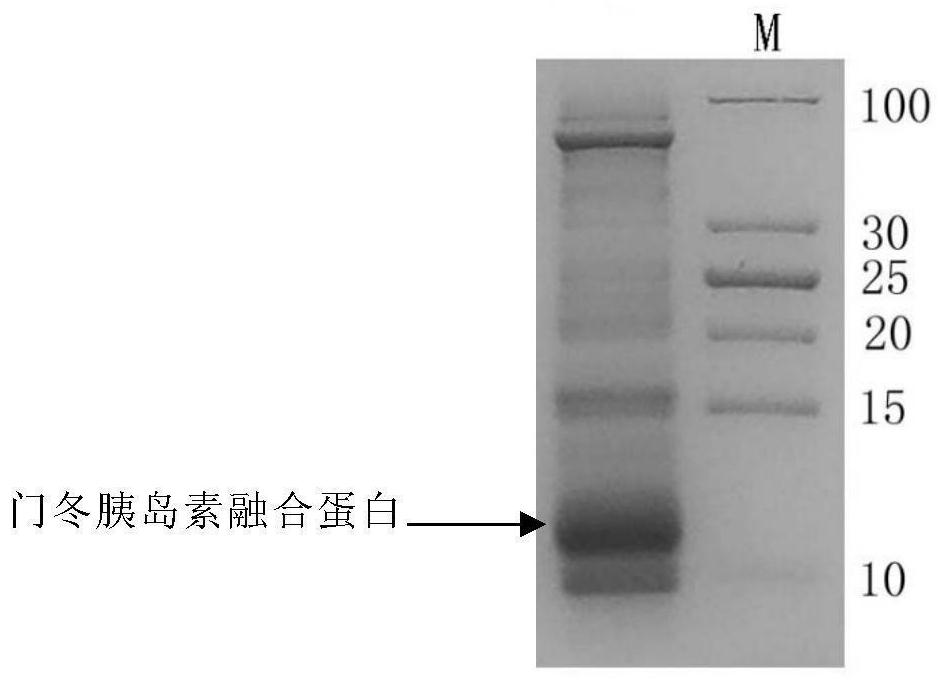
[0197] image 3 The SDS-PAGE electrophoresis of the insulin aspart fusion protein after the inclusion body denaturation is shown.
Embodiment 3
[0198] Embodiment 3 Enzymatic cleavage of fusion protein
[0199] Add dilute hydrochloric acid to the refolding solution to adjust the pH to 8.0-9.5, add recombinant trypsin at 1:3000, add carboxypeptidase B at 1:15000, enzymatic digestion temperature is 36-38°C, enzyme digestion time is 14-20h, Boc-insulin aspart obtained after enzyme digestion.
[0200] After 16 hours of enzyme digestion, the content of Boc-insulin aspart in the digestion solution was detected by HPLC. When the difference between the concentrations of Boc-insulin aspart detected for two consecutive hours was less than 3%, the enzyme digestion was completed. Finally, the concentration of Boc-insulin aspart in the digestion solution is 0.4-0.6 g / L, and the digestion rate is above 80%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com