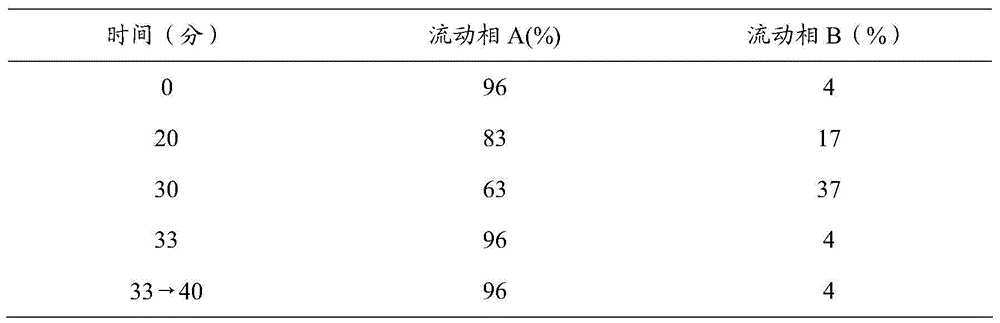
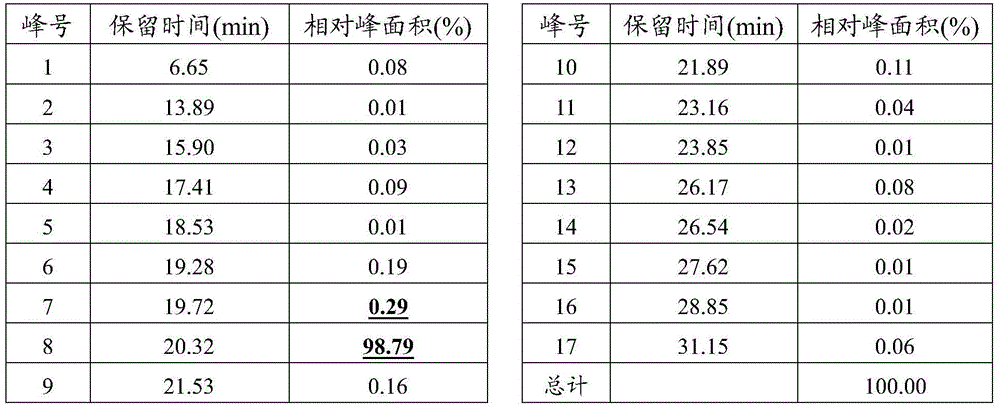
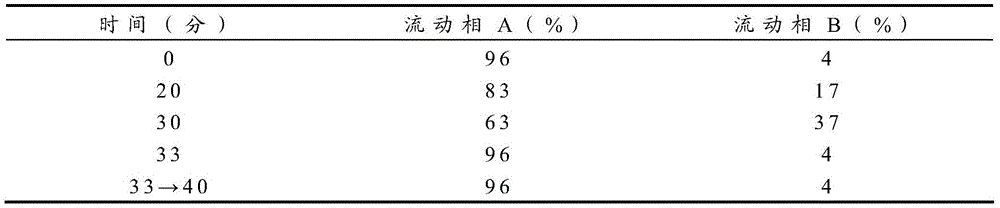
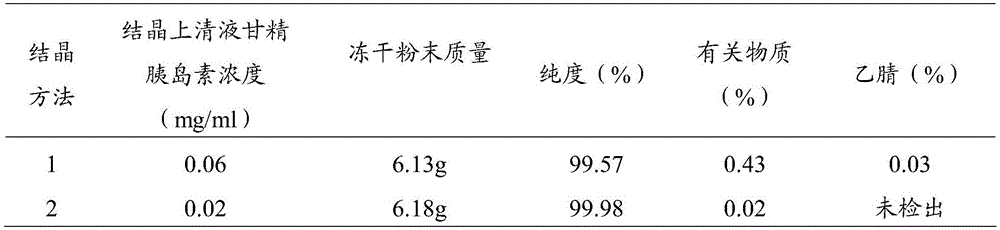
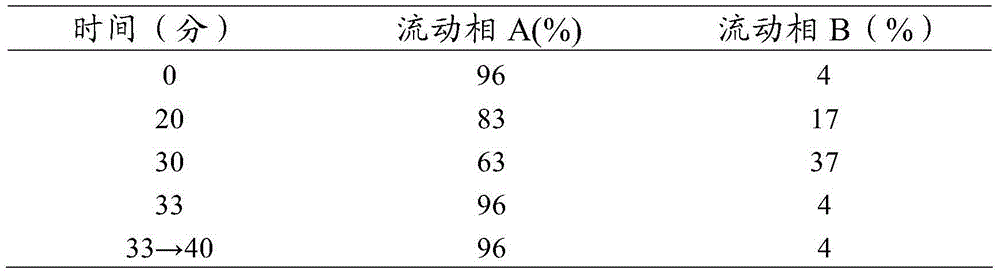
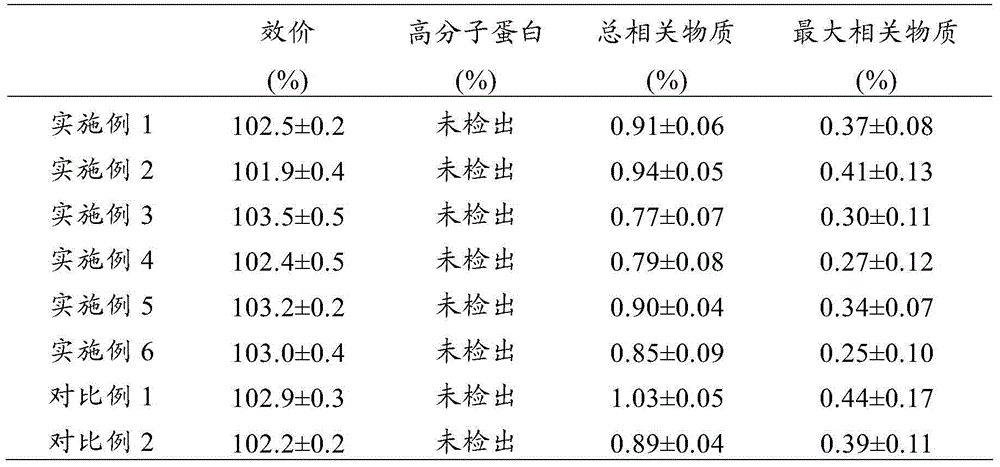
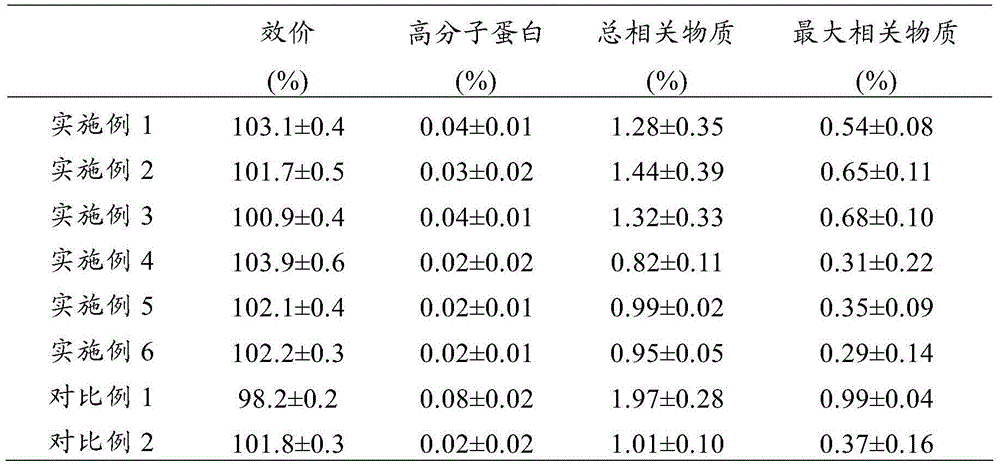
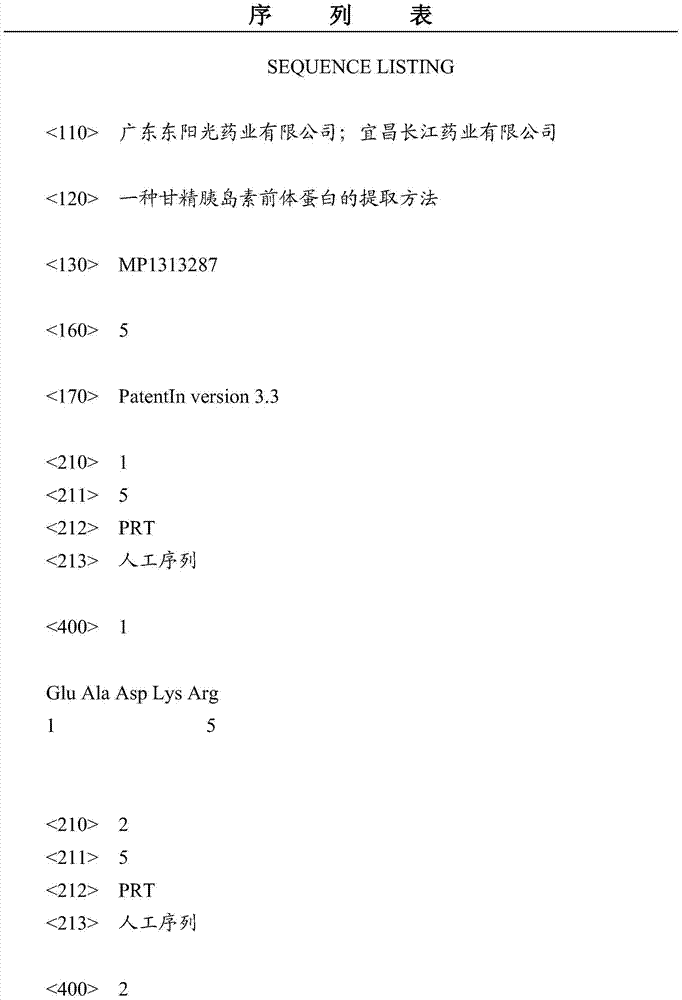
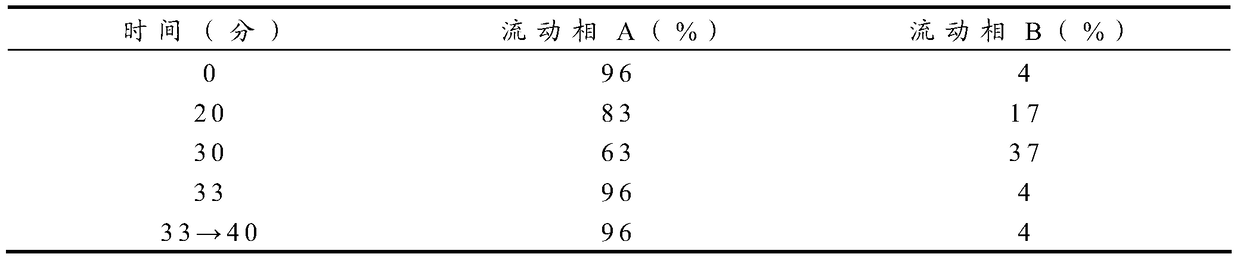
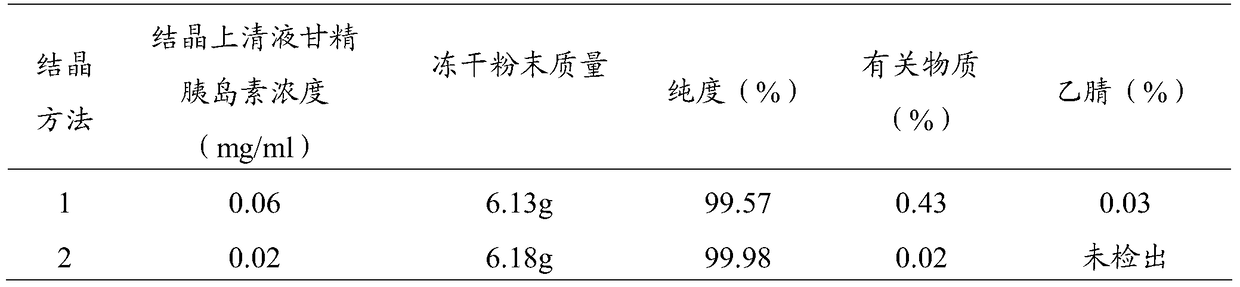
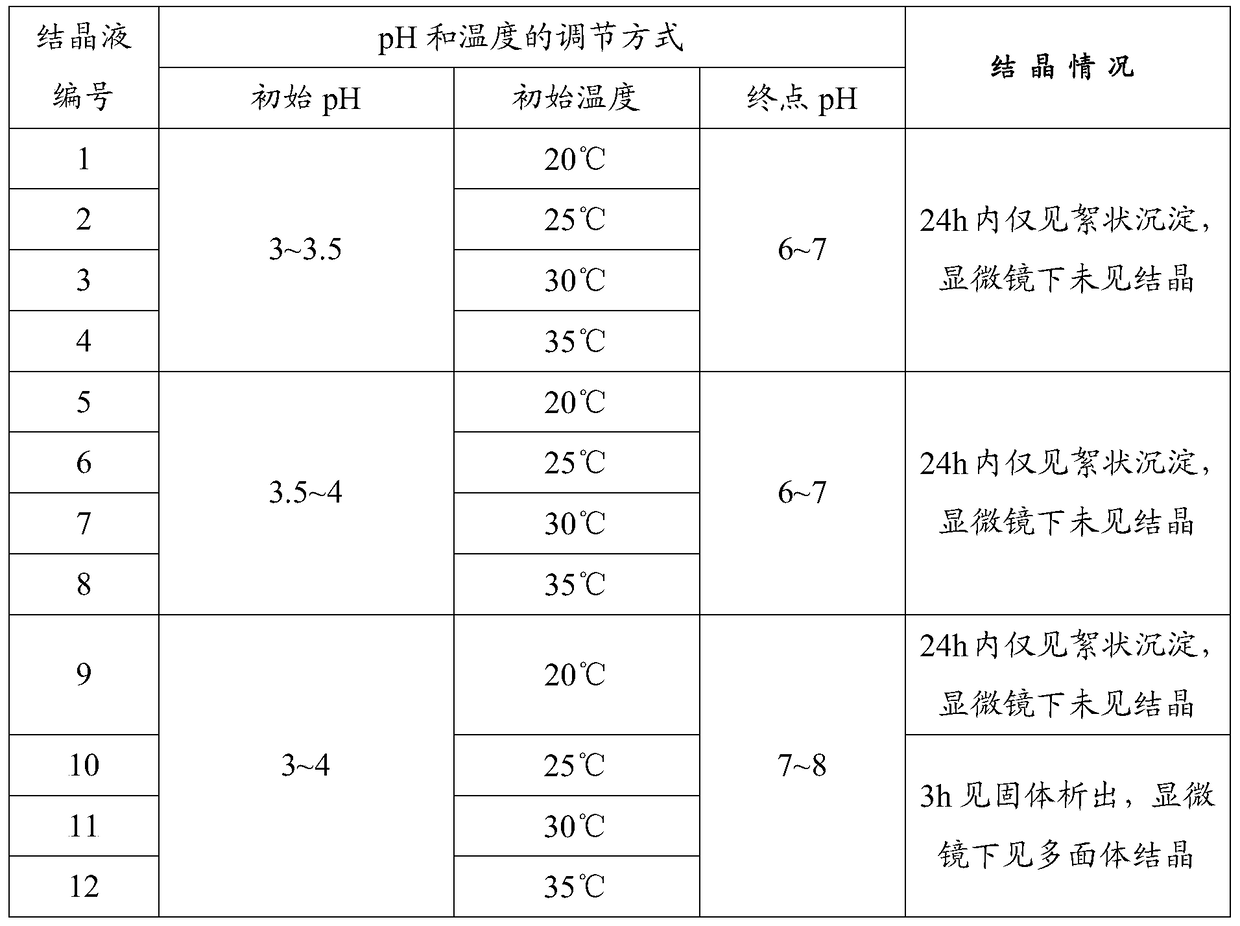
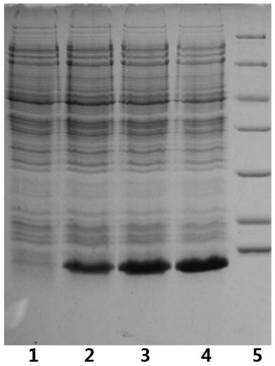
Patents
Literature
46 results about "Insulin glargine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Insulin glargine is used with a proper diet and exercise program to control high blood sugar in people with diabetes.
Method of treating diabetes type 2 by metformin and an ultrarapid acting insulin
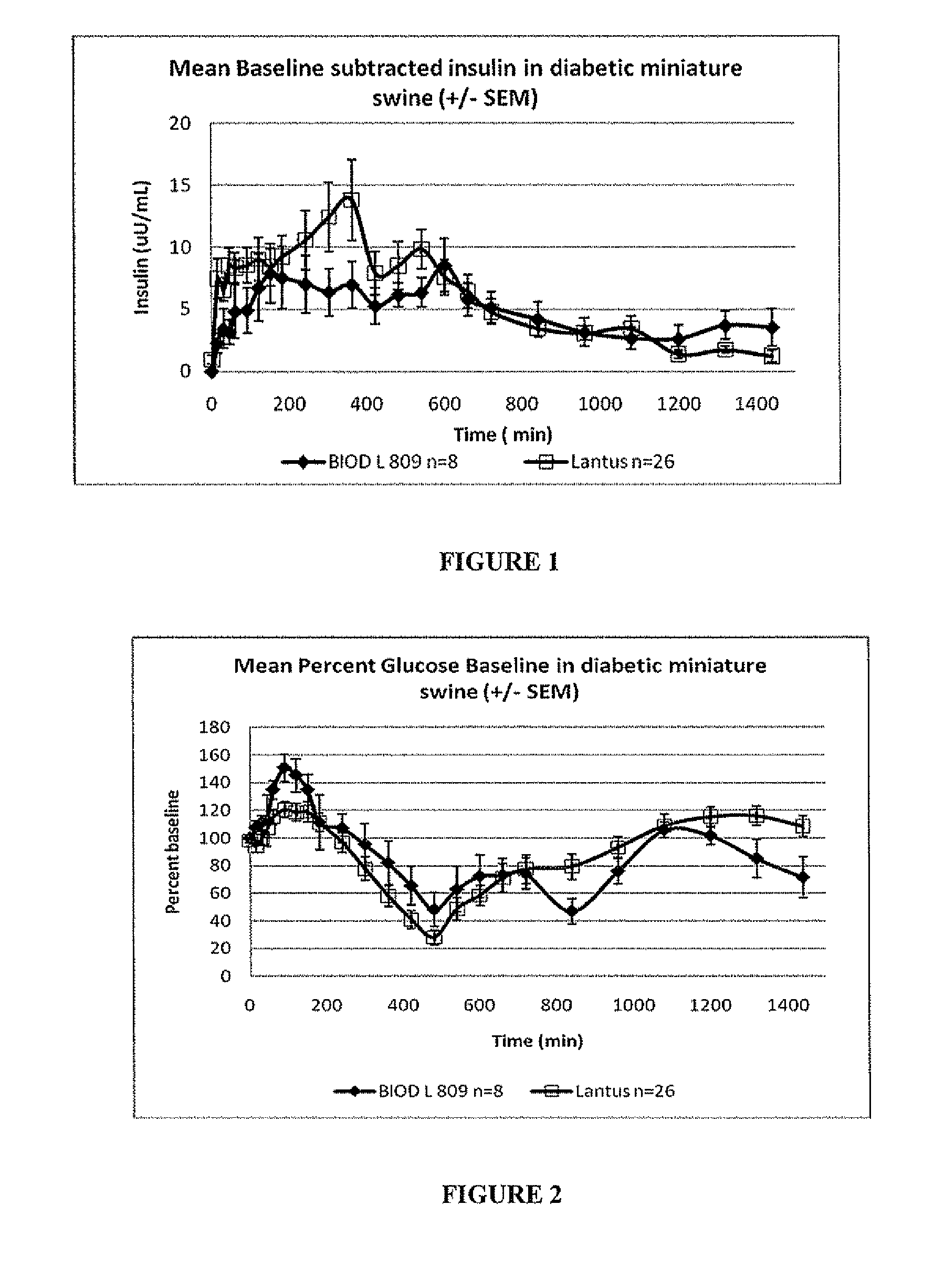
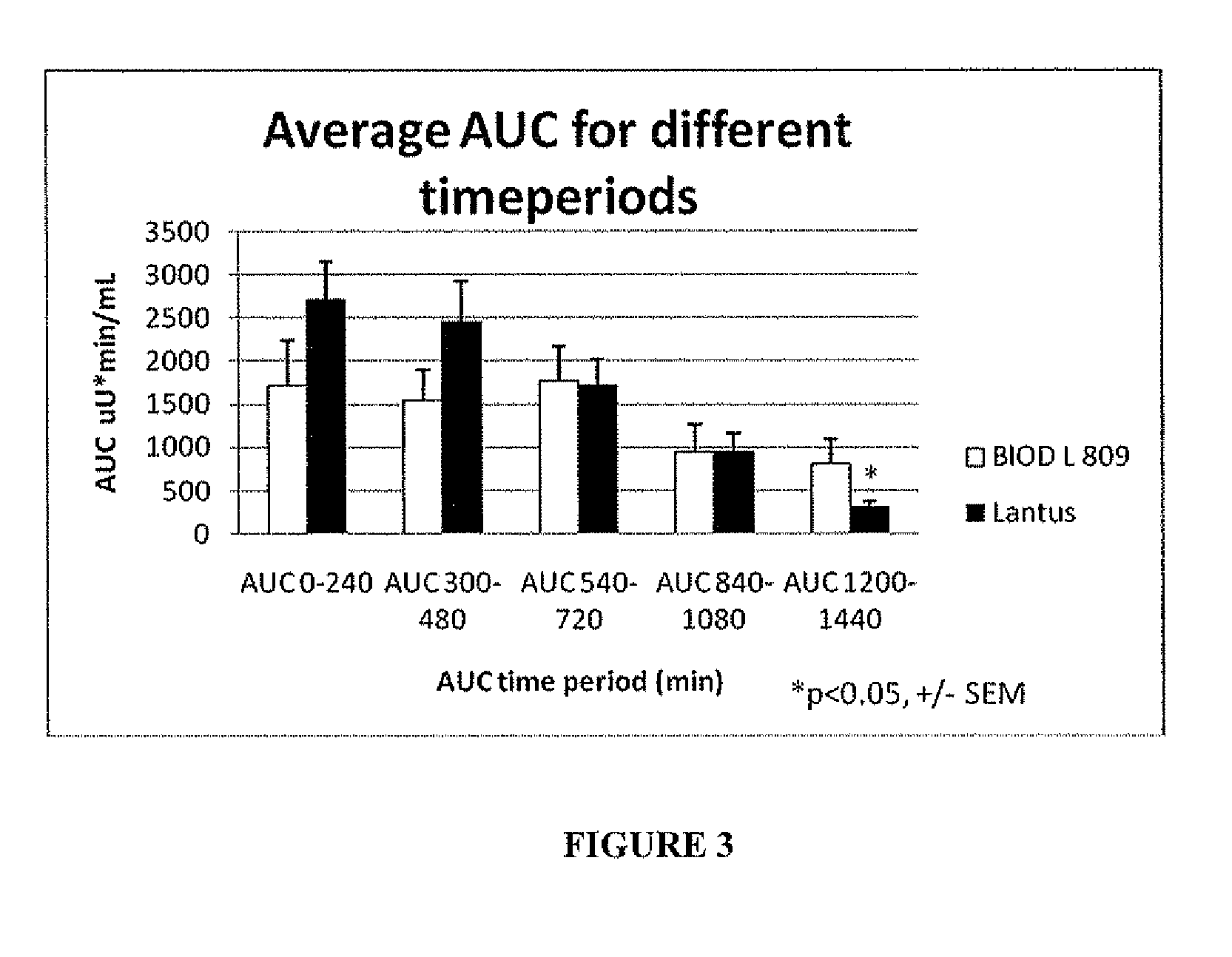
Disclosed herein are improved methods of treating hyperglycemia with a combination of an ultrarapid acting insulin and insulin glargine comprising prandial administration of the ultrarapid insulin, and administration of a first dose of insulin glargine within 6 hours of waking for a day.
Owner:MANNKIND CORP
Insulin with a stable basal release profile
ActiveUS20110281790A1Accelerated precipitationLess solublePeptide/protein ingredientsMetabolism disorderInsulin glargineZinc
A basal insulin formulation composed of insulin, preferably insulin glargine, injectable zinc and injectable iron compounds as precipitating and / or stabilizing agents has been developed for subcutaneous, intradermal or intramuscular administration. The formulation is designed to form a precipitate of insulin following injection, creating a slow releasing “basal insulin” over a period of 12 to 24 hours.
Owner:ELI LILLY & CO
Use of ultrarapid acting insulin
Disclosed herein are improved methods of treating hyperglycemia with a combination of an ultrarapid acting insulin and insulin glargine comprising prandial administration of the ultrarapid insulin, and administration of a first dose of insulin glargine within 6 hours of waking for a day.
Owner:MANNKIND CORP
Preparation method of insulin glargine and analogue thereof
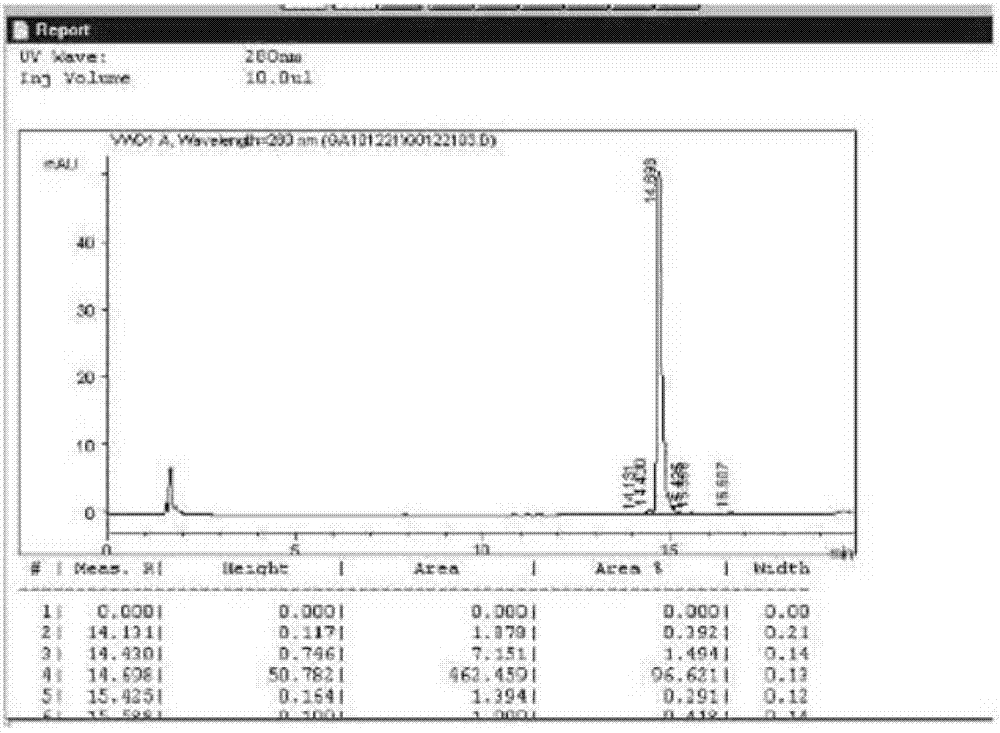
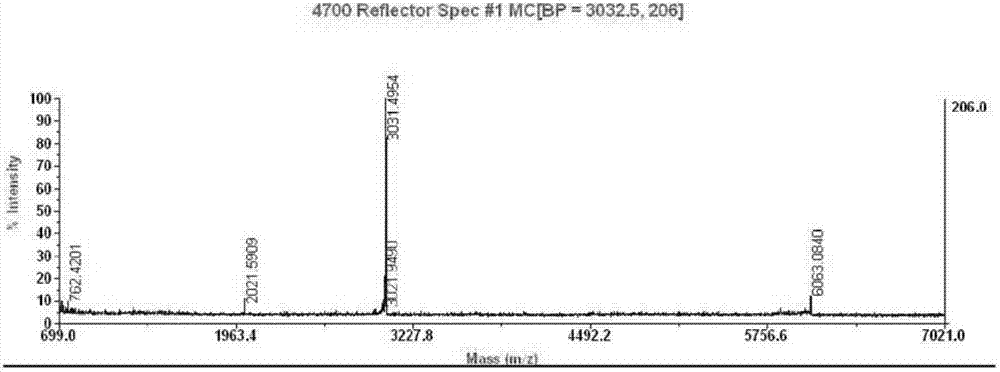
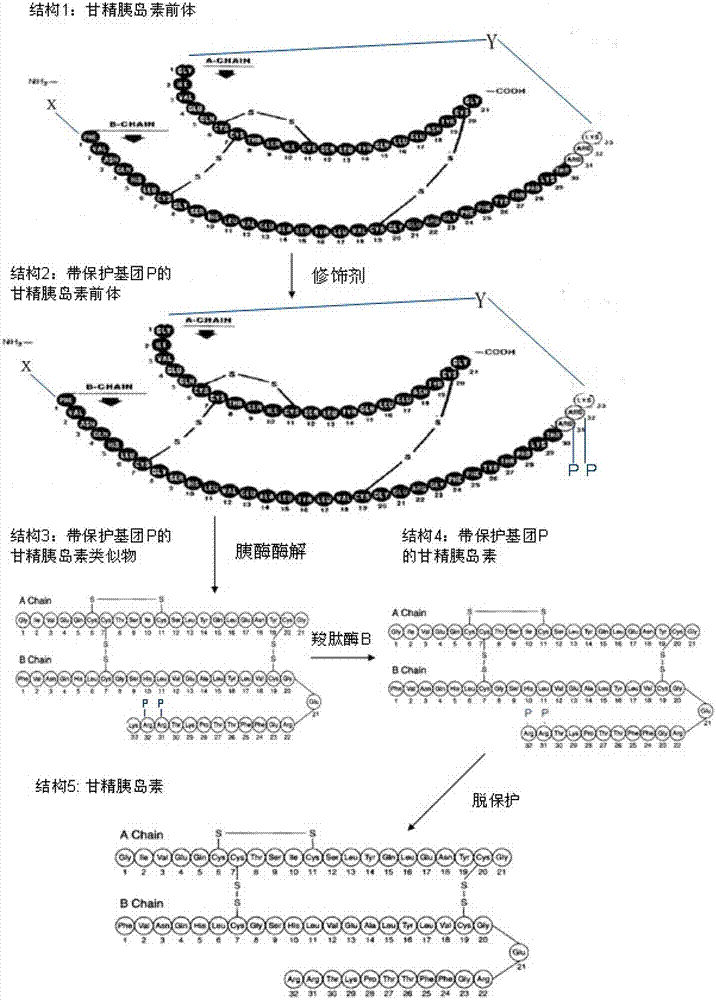
The invention discloses a preparation method of insulin glargine and an analogue thereof. The preparation method includes the following steps: (1) a gene engineering method is used for preparing a precursor of the insulin glargine and the analogue of the insulin glargine with a chain B and an end C containing a plurality of basic amino acids; (2) an amino acid side chain protective agent is used for distinguishing arginine or lysine through pancreatic enzyme specificity, and the insulin glargine and the analogue of the insulin glargine are provided with protecting groups and obtained under the effect of the protective agent and pancreatic enzyme; or the specificity is used for acting on clostripain of the arginine (Arg) or endoproteinase lysine (Lys) C of the Lys directly without protection; (3) carboxypeptidase is added optionally to remove unprotected basic amino acids at the tail end of the C; and (4) the glargine and the analogue of the insulin glargine are obtained through deprotection. The preparation method is simple and convenient, high in yield, wide in application range and suitable for introduction of more than two basic amino acids.
Owner:SHANGHAI HUAYI BIO LAB CO LTD
Insulin glargine injecta and preparation method thereof
ActiveCN102188367AQuality improvementImprove securityPeptide/protein ingredientsMetabolism disorderPreservativeInsulin injection
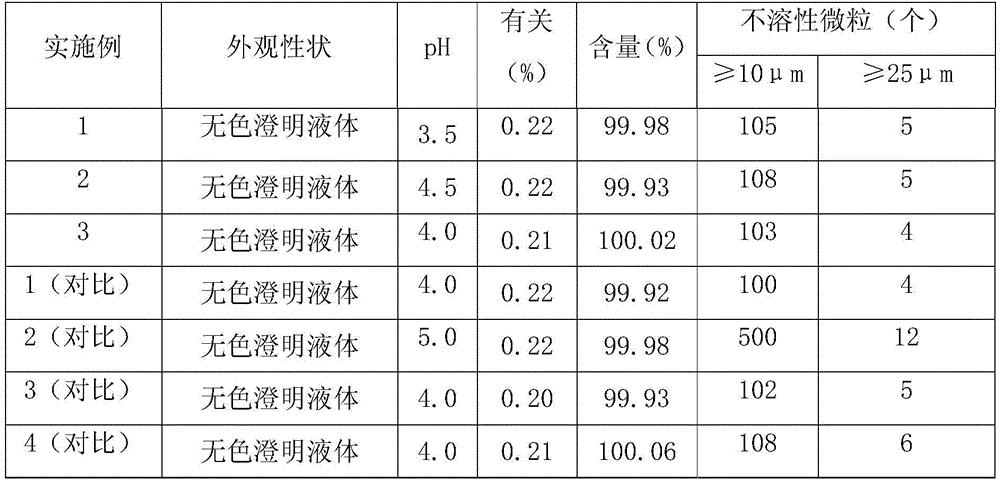
The invention relates to insulin glargine injecta and a preparation method thereof. Besides zinc and preservative, the insulin glargine injecta also comprises 0.5 to 50 percent (w / v: weight in volume) of PEG (polyethylene glycol). A pH value of the insulin glargine injecta is regulated into the range of 3.8 to 4.2 by using citric acid. The preparation method of the insulin glargine injecta comprises the following steps of: firstly adding insulin glargine into water for injection, regulating the pH value into the range of 3.5 to 4.5 by using the citric acid and dissolving the insulin glargine; then adding the PEG to obtain a solution I; adding the zinc and the preservative into the rest of water for injection, stirring to dissolve the zinc and the preservative and regulating the pH value into the range of 3.5 to 4.5 by using the citric acid to obtain a solution II; and mixing the solution I and the solution II and filtering and degerming the mixed solution to obtain the insulin glargine injecta. The medicinal insulin glargine injecta provided by the invention has the advantages of stable performance, rapid response and high safety.
Owner:鲁南新时代生物技术有限公司
Method of obtaining a purified, biologically active heterologous protein
ActiveUS8802816B2Polypeptide with localisation/targeting motifPeptide/protein ingredientsHeterologousOrganism
The invention relates to methods of separation and / or purification of impurities yielding a purified heterologous protein product devoid of related impurities or with substantially minimal quantities of such glycosylated impurities. More specifically, the invention relates to the identification of glycosylated forms of insulin analogues such as glargine impurities characterized post expression in yeast based systems such as Pichia pastoris. The invention also relates to methods used to clone gene encoding the protein insulin glargine; inserting the related gene in a suitable yeast host; producing culture of the recombinant strain, stimulating expression of the heterologous polypeptide, its secretion and purification post fermentation and related enzymatic conversions.
Owner:BIOCON LTD
Preparation method of insulin glargine injection and insulin glargine injection prepared by using preparation method
ActiveCN104688678AQuality improvementProcess impurity reductionPeptide/protein ingredientsMetabolism disorderMedicineInsulin injection
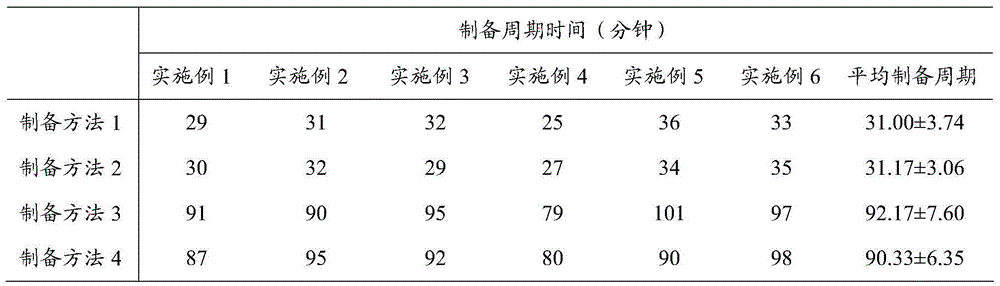
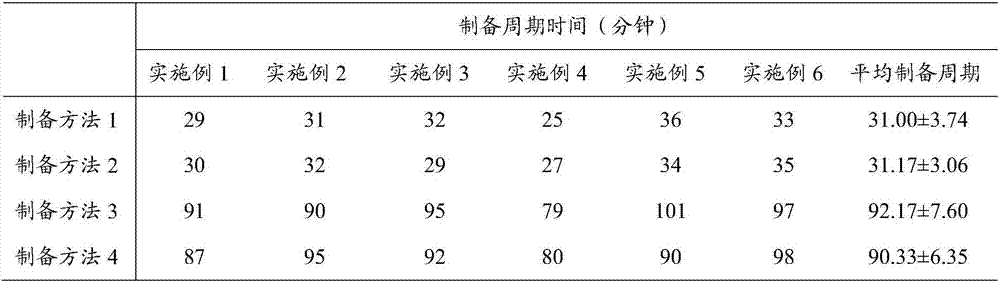
The invention provides a preparation method of an insulin glargine injection and the insulin glargine injection prepared by using the preparation method. The preparation method comprises the following steps: dissolving glycerol into part of water for injection to prepare a glycerol solution, then dividing the glycerol solution into three parts, and respectively adding insulin glargine, metacresol and zinc chloride; firstly uniformly mixing an insulin glargine-glycerol solution and a metacresol-glycerol solution to obtain a mixed solution I; then adding a hydrochloric acid solution into the mixed solution I until the pH value is equal to 3.0 to 3.5, then adding a zinc chloride-glycerol solution, uniformly stirring, and then adding a sodium hydroxide solution until the pH value is equal to 3.5 to 4.5; and finally stabilizing the volume to reach a final volume by using the water for injection. By adopting the preparation method provided by the invention, insulin glargine and other auxiliary materials can be rapidly dissolved, the preparation cycle time can be significantly shortened, process impurities produced in a preparation process can be reduced, the quality of the insulin glargine injection can be improved, the process energy consumption can also be reduced, and the production efficiency can be improved; and the preparation method can be more suitable for the requirements of large-scale production.
Owner:TONGHUA DONGBAO PHARMA
Preparation method of insulin glargine and insulin glargine prepared by same
ActiveCN105585628AReduce contentHigh yieldPeptide preparation methodsInsulinsOrganic acidInsulin glargine
The invention provides a preparation method of insulin glargine and insulin glargine prepared by the same. With an insulin glargine solution as a raw material, the method comprises the following steps: mixing the insulin glargine solution, an organic acid, a phenol derivative, a zinc salt and water to obtain crystalline liquid; adjusting pH to 3-4 and preserving heat for 1-8 hours at 25-35 DEG C; adjusting pH to 7.0-8.0 and cooling to 2-8 DEG C; standing for 3-5 hours; centrifuging; and separating the solid and supernate. Compared with prior art, the preparation method provided by the invention realizes higher production efficiency and product quality so as to better guarantee the long-term medication safety of the patients.
Owner:TONGHUA DONGBAO PHARMA
Use of ultrarapid acting insulin
Disclosed herein are improved methods of treating hyperglycemia with a combination of an ultrarapid acting insulin and insulin glargine comprising prandial administration of the ultrarapid insulin, and administration of a first dose of insulin glargine within 6 hours of waking for a day.
Owner:MANNKIND CORP
Method for improving efficiency in preparation of insulin and like
ActiveCN105294854AFacilitates linear scalingHuge industrial application valuePeptide preparation methodsInsulinsEnzyme digestionInsulin glargine
The invention provides a method for improving the efficiency in preparation of insulin and the like. The method comprises the step of conducting chromatography on a precursor of insulin glargine by use of a hydroxyapatite medium. The invention further provides a preparation method for active insulin glargine. The preparation method comprises the step of conducting enzyme digestion on a recombinant expression precursor of insulin glargine by use of a Kex-2p enzyme. In a preferred embodiment of the invention, the preparation method comprises the following steps: obtaining the recombinant expression precursor of insulin glargine; conducting enzyme digestion on the recombinant expression precursor of insulin glargine by use of the Kex-2p enzyme; conducting further chromatographic purification on the enzyme digestion product, so as to obtain active insulin glargine.
Owner:烟台普罗吉医药科技有限公司 +1
Enzyme digestion transformation method of insulin glargine precursor
InactiveCN102994600AEasy to purifyHigh enzyme digestion yieldFermentationEnzyme digestionInsulin glargine
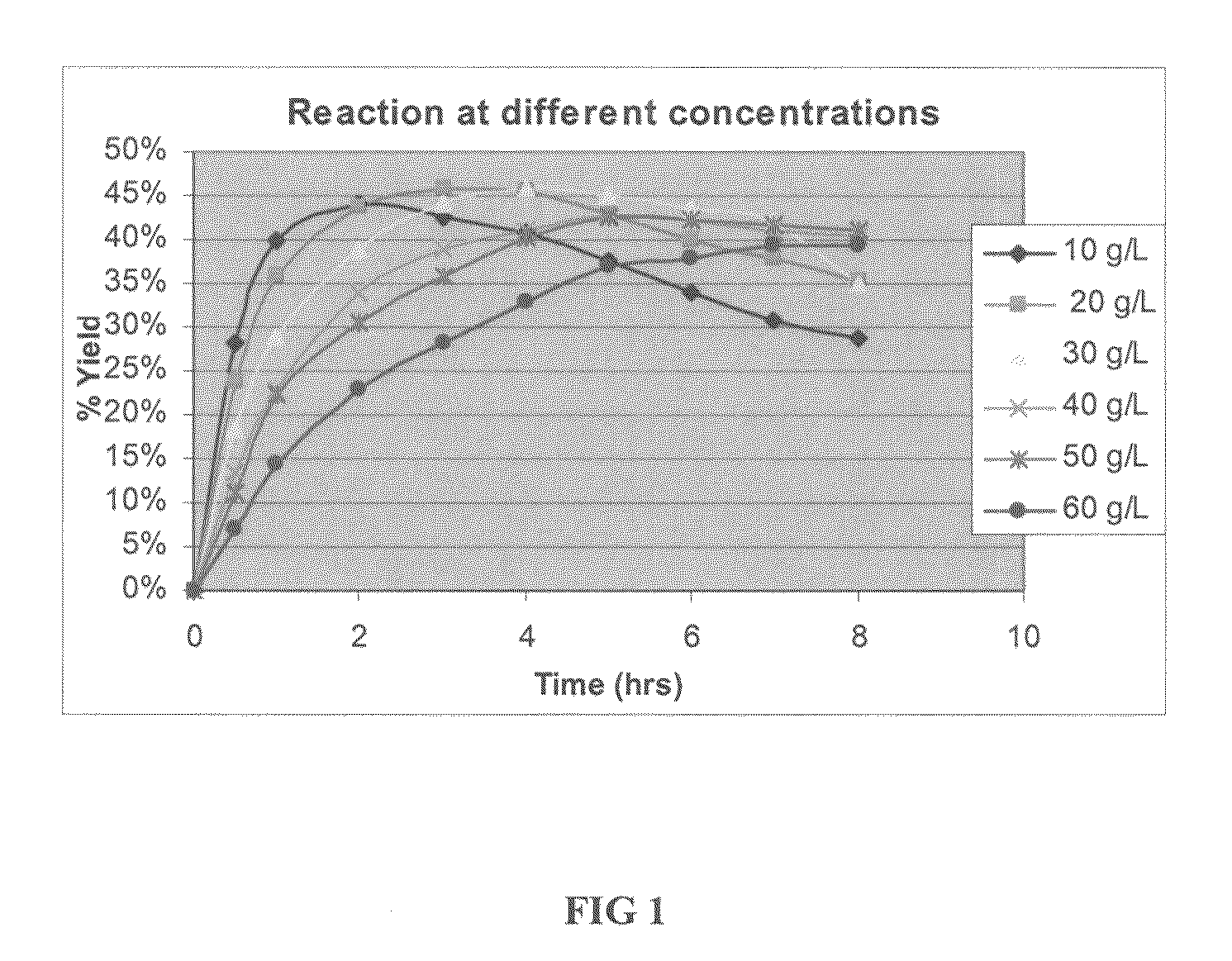
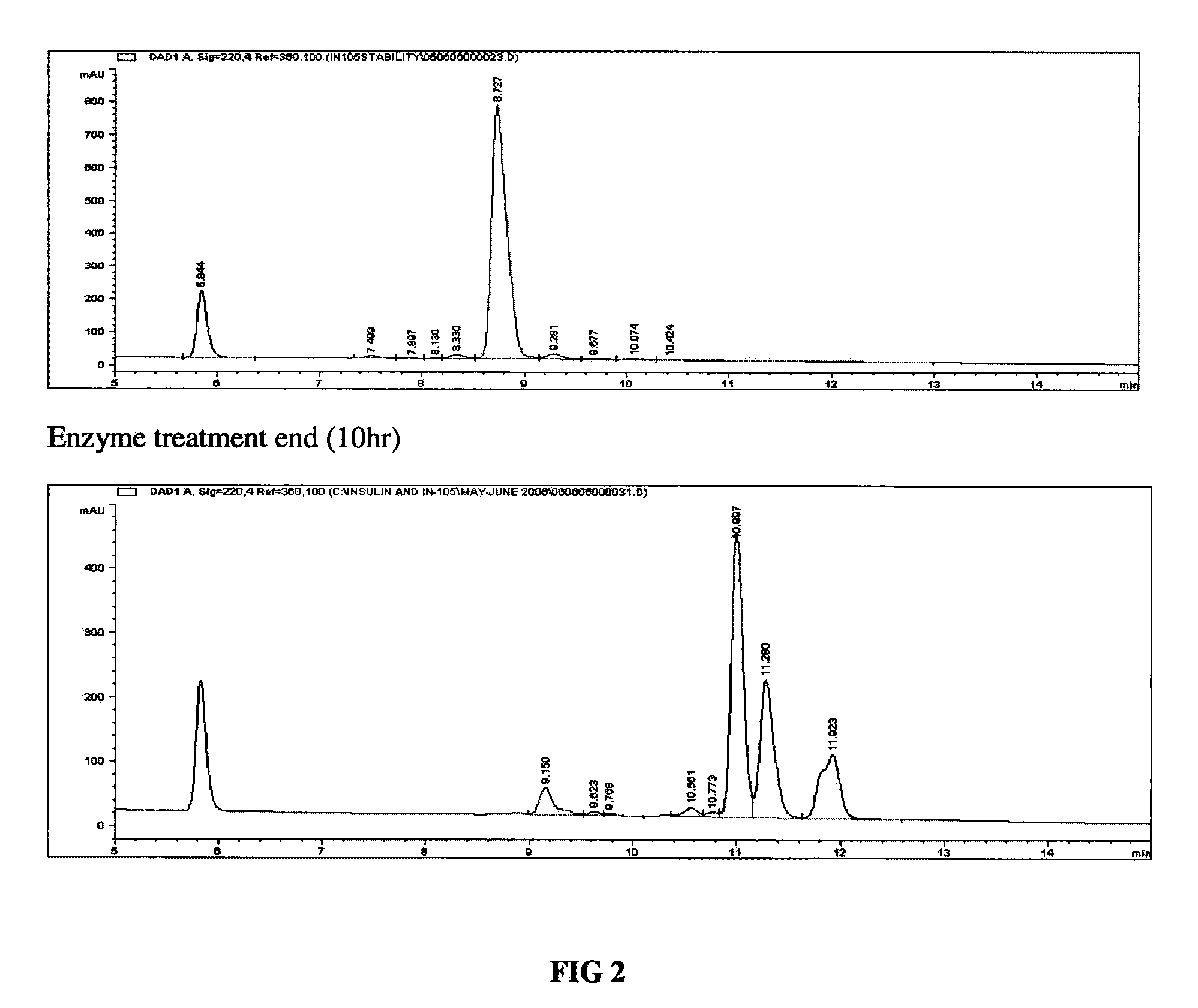
The invention discloses an enzyme digestion transformation method of an insulin glargine precursor. The method comprises the following steps: dissolving the insulin glargine precursor in a buffer solution with pH of 8-11; controlling a temperature of a reaction system at 0-37 DEG C; adding trypsin according to a mass ratio of trypsin to insulin glargine precursor being 1:1000-10000; and reacting for 2-40 h. The enzyme digestion transformation method provided by the invention can significantly increase the amount of produced insulin glargine, while significantly reducing by-product, has high enzyme yield and easy purification of the enzyme digestion solution, and is suitable for industrial production.
Owner:鲁南新时代生物技术有限公司
External biological activity determination method for human insulin and analog or conjugate
ActiveCN105092490AGood repeatabilityEasy to operateMaterial analysis by observing effect on chemical indicatorColor/spectral properties measurementsPEGylated insulinDrug biological activity
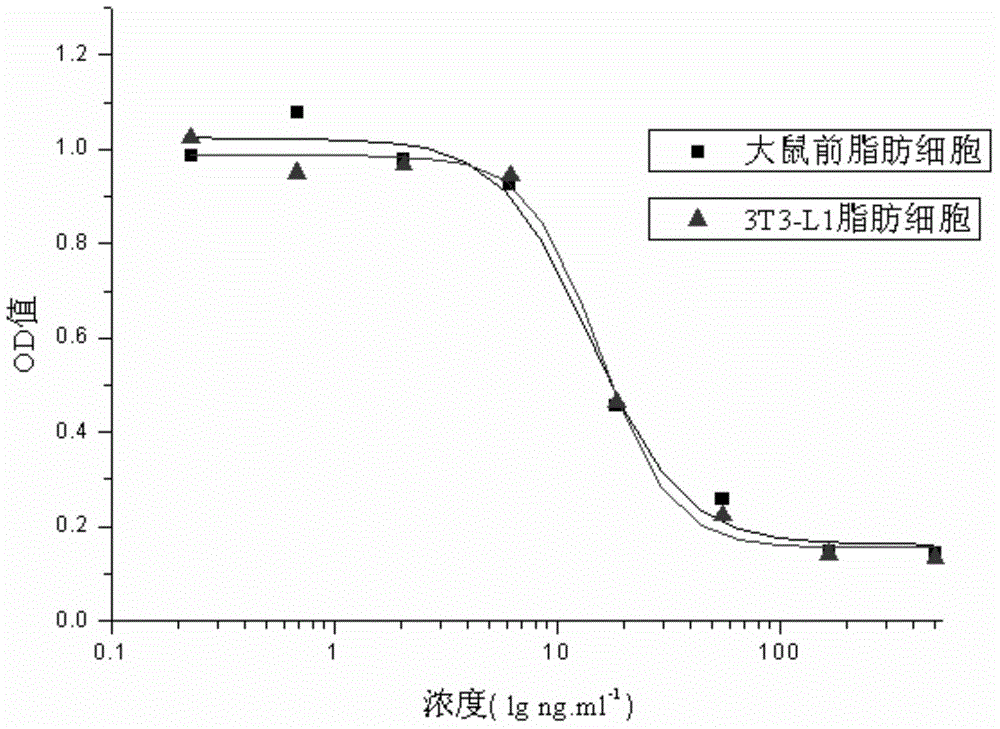
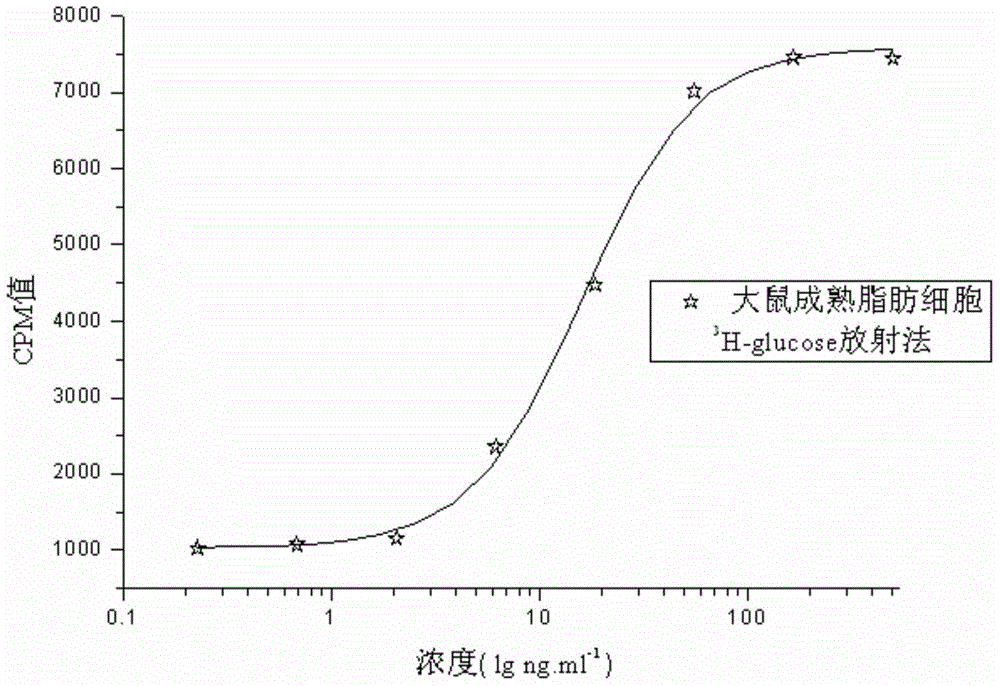
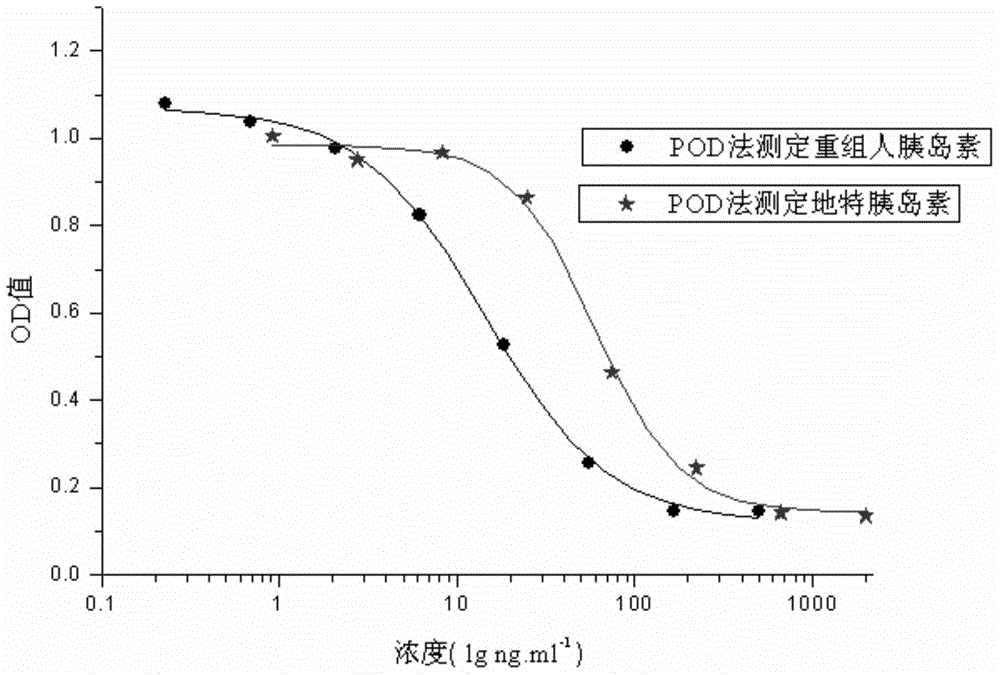
The invention relates to a human insulin and analog including rapid-acting insulin (e.g. Insulinaspart, InsulinLispro, and InsulinGlulisine), long-acting insulin (e.g. Insulinglargine, InsulinDetemir, and InsulinDegludec), and modified conjugate (e.g. PEG-Insulin and PEG conjugatedinsulin lispro ), and also relates to the biological value determination method for the cell culture in vitro of PEG-Insulin and application thereof. The external biological activity determination method for human insulin and analog or conjugate is induced in vitro into adipocyte by using preadipocyte of male rat, and measure the glucose consumption after insulin reacting in the adipocyte nutrient solution by using glucose oxidase method to calculate the corresponding insulin biological value.
Owner:CHONGQING PEG BIO BIOTECH CO LTD
Insulin with a stable basal release profile
ActiveUS8637458B2Accelerated precipitationLess solublePeptide/protein ingredientsMetabolism disorderInsulin glargineINSULIN PREPARATIONS
A basal insulin formulation composed of insulin, preferably insulin glargine, injectable zinc and injectable iron compounds as precipitating and / or stabilizing agents has been developed for subcutaneous, intradermal or intramuscular administration. The formulation is designed to form a precipitate of insulin following injection, creating a slow releasing “basal insulin” over a period of 12 to 24 hours.
Owner:ELI LILLY & CO
Culture medium and method for producing insulin glargine precursor by fermenting with culture medium
InactiveCN104726524ALow costEasy to operateMicroorganism based processesFermentationEscherichia coliMonopotassium phosphate
The invention relates to a culture medium and a method for producing an insulin glargine precursor by fermenting with the culture medium. The invention is characterized in that 1 liter of the culture medium comprises 3-8g of citric acid, 0.01-0.1g of ferric sulfate, 2-8g of diammonium hydrogen phosphate, 2-4g of monopotassium phosphate, 1-4g of magnesium sulfate, 8-15g of glucose, 1-3g of glycerinum, 10-16g of yeast extracts, 0.05-0.2g of vitamin B1, 0.5-1mg of trace element ammonium molybdate, 0.1-1mg of copper sulfate, 1-4mg of boric acid, 0.2-0.6mg of potassium iodide, 1-4mg of manganese chloride, 1-5mg of zinc acetate and the balance of water. The method comprises the steps that a recombinant escherichia coli BL21(DE3) / hp1 strain is cultured by using a solid slant culture medium to obtain a monoclone; the monoclone is inoculated onto a liquid seed culture medium to be cultured; the obtained liquid seed is inoculated on the culture medium; the insulin glargine precursor is prepared by oscillation fermentation culture by a shake flask or fermentation culture in a ventilation stirring fermentation tank. The method is easy to operate and low in cost of raw materials; the protein content of the obtained insulin glargine precursor reaches 7g / l at least and 11g / l at most, and a new path is opened up for large-scale production of the insulin glargine.
Owner:麦科罗夫(南通)生物制药有限公司
A method for preparing insulin glargine crystals
ActiveCN106117345BGood crystal formEasy to operatePeptide preparation methodsInsulinsOrganic acidGlycine
Owner:YICHANG HEC CHANGJIANG PHARMA CO LTD
Renaturation method of insulin glargine precursor
ActiveCN103694339ASimple purification processShort reaction timePeptide preparation methodsInsulinsInsulin glargineCombinatorial chemistry
The invention discloses a renaturation method of an insulin glargine precursor, and belongs to the field of biomedical protein folding. The method comprises the following steps: dissolving the insulin glargine precursor in a modifier solution, adding a reducer for reduction, adjusting the pH value to be 9.5-11.5, controlling the temperature of a reaction system to be 35-45 DEG C, and reacting for 30-60 minutes to obtain a modified insulin glargine precursor solution; adding the modified insulin glargine precursor solution into a dilute buffer solution, adding a protein folding additive, adjusting the pH value to be 9.5-11.5, continuously inletting air into the solution, controlling the temperature of the reaction system to be 0-20 DEG C, and reacting for 2-40 hours to obtain an insulin glargine renaturation solution. According to the method, the renaturation reaction time is shortened, the correctly folded protein content is increased, the renaturation efficiency is improved to be 51%-62%, the production cost is reduced, and the large-scale industrialization production and application are facilitated.
Owner:ZHUHAI UNITED LAB
Method for preparing insulin glargine crystal
ActiveUS9187520B2Quality improvementEasy to preparePolycrystalline material growthFrom normal temperature solutionsOrganic acidOrganic solvent
Disclosed is a method for preparing an insulin glargine (GlyA21-ArgB31-AryB32-human insulin) crystal, comprising crystallizing the insulin glargine at pH 7.0-9.0 and in a crystallization solution containing a recombinant insulin glargine, an organic solvent of a 10-30% concentration by volume, a zinc compound, a phenol derivative, a salt and an organic acid.
Owner:GAN&LEE PHARMA
Stable insulin glargine injection and preparation method thereof
ActiveCN104688677AImprove stabilityGuarantee safe and effectivePeptide/protein ingredientsPharmaceutical delivery mechanismMetacresolInsulin injection
The invention discloses a stable insulin glargine injection and a preparation method thereof. The stable insulin glargine injection comprises the following components: 100IU / mL of insulin glargine, 10-100 mu g / mL of zinc, 1.5-3.5 mg / mL of metacresol, 0.7-1.8 mg / mL of phenol, 15-20 mg / mL of glycerin, and hydrochloric acid and / or sodium hydroxide and injection water, the pH value is 3.8-4.2. By means of modifying the raw material components and the preparation process of the insulin glargine injection, the stability of the insulin glargine injection is increased and the quality of the preparation is improved, and the insulin glargine injection does not have allergic reactions and the safety is good.
Owner:TONGHUA DONGBAO PHARMA
Extraction method of insulin glargine precursor protein
ActiveCN103833828AHigh protein contentIncrease productivityPeptide preparation methodsProtein targetCentrifugation
The invention relates to the field of biochemistry and discloses an extraction method of an insulin glargine precursor protein. The extraction method comprises the following steps of adding an acid into an insulin glargine precursor protein fermentation broth to adjust a pH value to 1-4, carrying out centrifugation and collecting a supernatant so that the insulin glargine precursor protein is obtained. Before bacterium centrifugation, the acid is used to adjust a pH value of the insulin glargine precursor protein fermentation broth to 1-4 so that after extraction, insulin glargine precursor protein content is improved, target protein loss caused by direct centrifugation removal of the bacteria is avoided and an insulin glargine yield is improved.
Owner:YICHANG HEC CHANGJIANG PHARMA CO LTD
Method for direct secretory expressing of mature double-chain insulin glargine by using saccharomyces cerevisiae
ActiveCN110331159AEasy to produceDemonstrate endonuclease activityPolypeptide with localisation/targeting motifFungiIon exchangeBiology
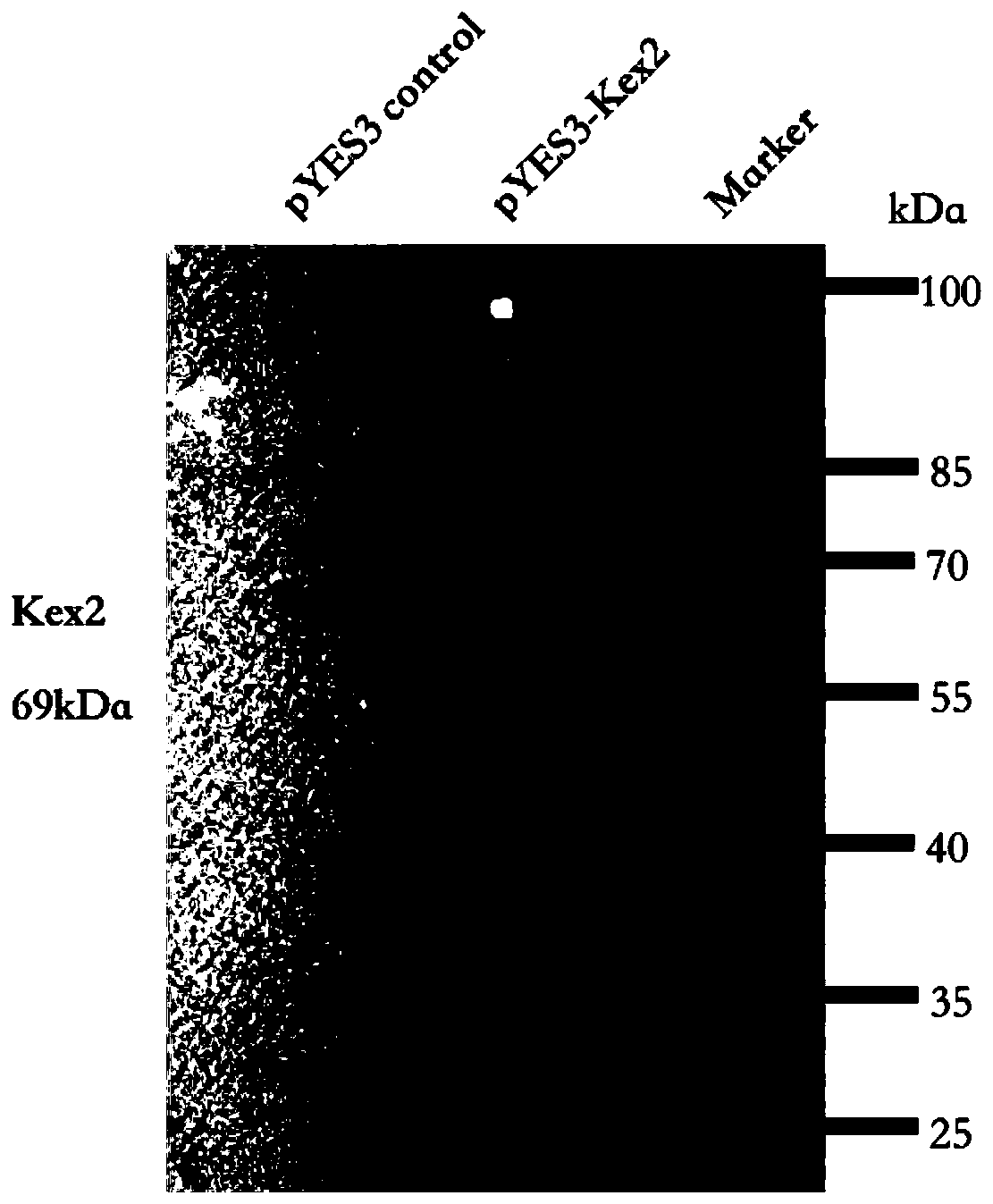
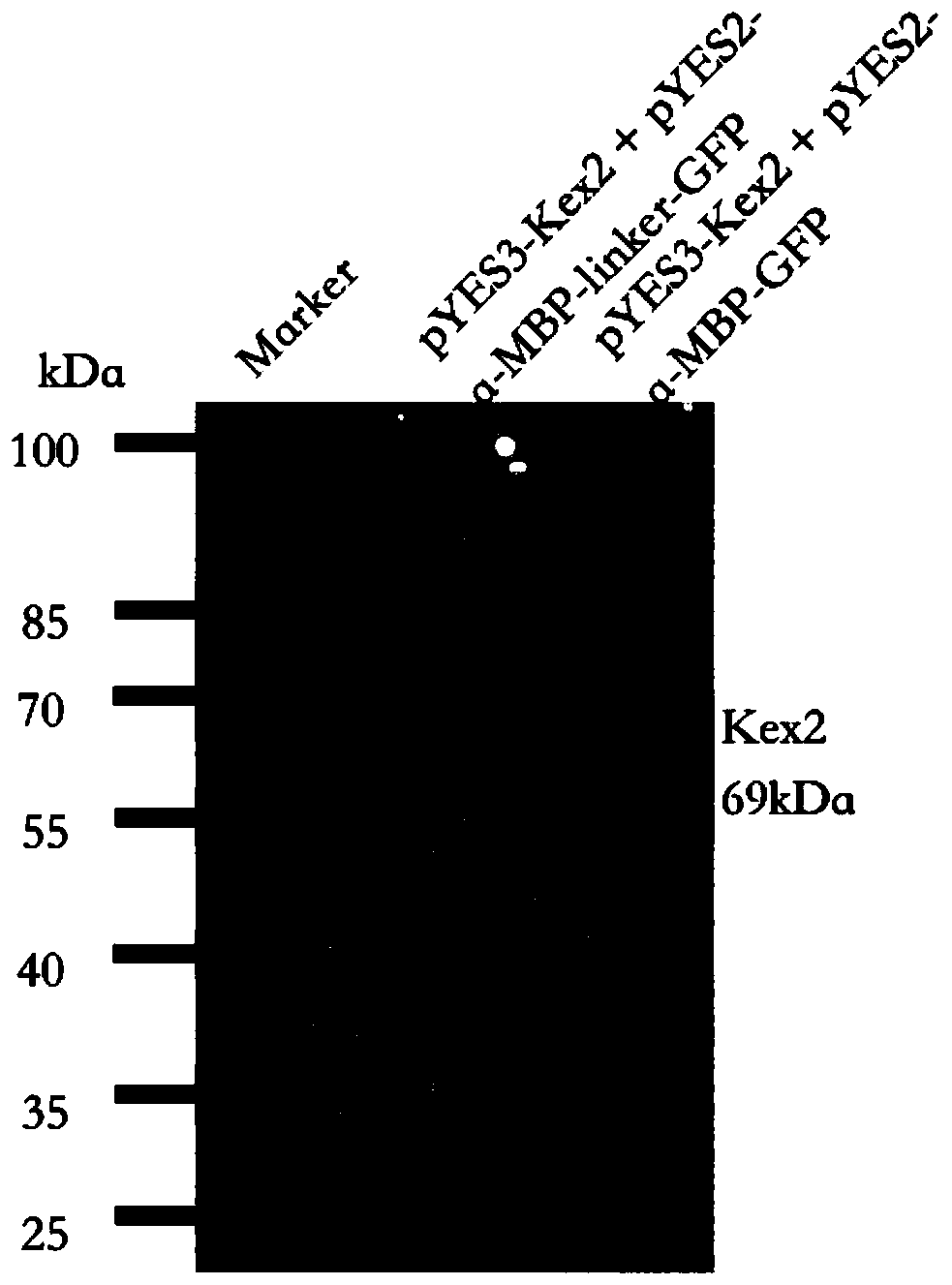
The invention discloses a method for direct secretory expressing mature double-chain insulin glargine by using saccharomyces cerevisiae. The method comprises the steps that firstly a chassis cell over-expressing Kex2 is constructed, then tandem signal peptide and codon-optimized insulin glargine DNA are transferred into a chassis cell over-expressing Kex2 to form a saccharomyces cerevisiae co-expression system for protein expression purification, and the insulin glargine is obtained. The method greatly reduces the difficulty of the production process of the insulin glargine, simplifies the downstream multi-step tedious and complicated processing flow of the traditional process, and enables yeast cells to directly perform the modification work in the cell. Meanwhile, the method reduces thecomplexity of purification, and the mature insulin protein can be directly extracted from a culture medium by relatively low-cost ion exchange purification. In addition, since the use of components such as trypsin is avoided, nearly 50% by-products in the original process are avoided, the fermentation use efficiency of nutrients such as carbon sources in the medium is improved, and the good application prospect is achieved.
Owner:SUN YAT SEN UNIV
Insulin glargine injection and preparation method thereof
ActiveCN105597087ASolve the phenomenon of white spotsImprove product qualityPeptide/protein ingredientsMetabolism disorderFiltrationInsulin injection
The invention relates to an insulin glargine injection. The insulin glargine injection is prepared from insulin glargine, glycerin, absolute ethyl alcohol, m-cresol, zinc chloride, benzalkonium chloride, PH regulator and water for injection. The insulin glargine injection is prepared through the following steps that the prescription dose of the insulin glargine is added into an appropriate amount of water for injection, 1 M of a hydrochloric acid solution is added while stirring is conducted, and the insulin glargine is dissolved, wherein the water for injection is precooled to room temperature; the prescription dose of m-cresol, zinc chloride and benzalkonium chloride are added, the mixture is stirred to be uniform, and the solution pH value is adjusted with the PH regulator; the prescription dose of glycerin and absolute ethyl alcohol are added to the scale volume, and the solution is stirred to obtain colorless clear liquid; filtration, filling and light inspection are conducted, and a finished product is obtained. Compared with the prior art, the phenomenon that white points occur in the insulin glargine injection is relieved; meanwhile, the product is stable in quality, and an obvious change does not exit after related substances are unpacked and put at room temperature and the related substances are stored for a long time at 2-8 DEG C.
Owner:SHANDONG NEWTIME PHARMA
Insulin glargine derivative and application thereof
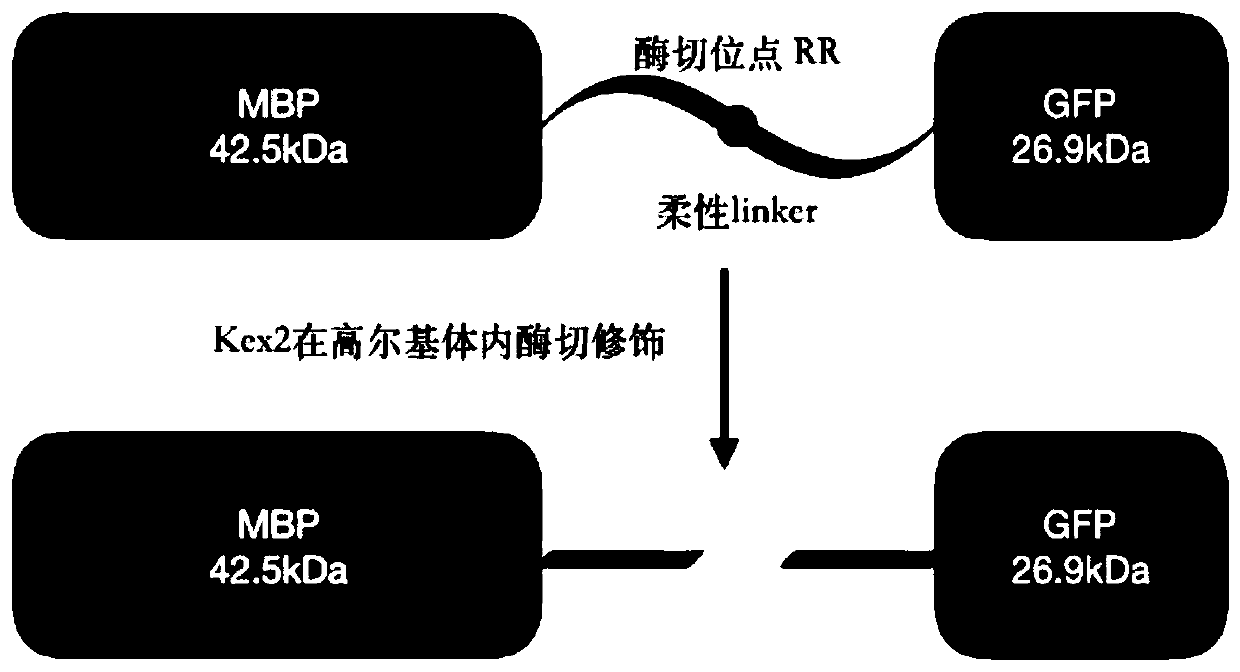
The invention provides an insulin glargine derivative and a preparation method thereof. Specifically, the invention provides a fusion protein comprising a green fluorescent protein folding unit and insulin glargine or an active fragment thereof. Expression quantity of the fusion protein disclosed by the invention is remarkably improved, and the insulin glargine protein in the fusion protein is correctly folded and has biological activity. Moreover, the green fluorescent protein folding unit in the fusion protein can be digested into small fragments by protease, and compared with the target protein, molecular weight difference is large, and separation is easy. The invention also provides a method for preparing insulin glargine by using the fusion protein and a preparation intermediate.
Owner:NINGBO KUNPENG BIOTECH CO LTD
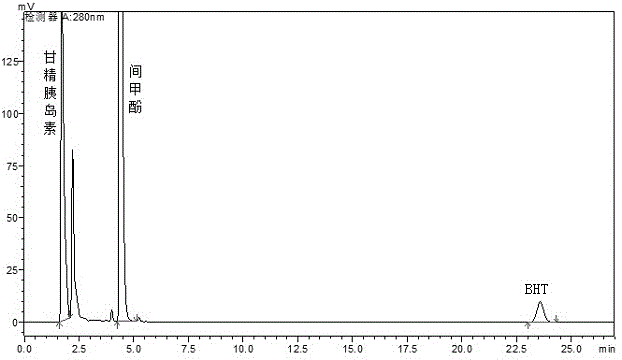
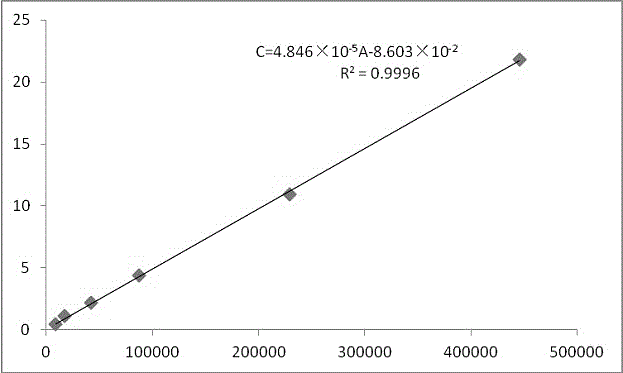
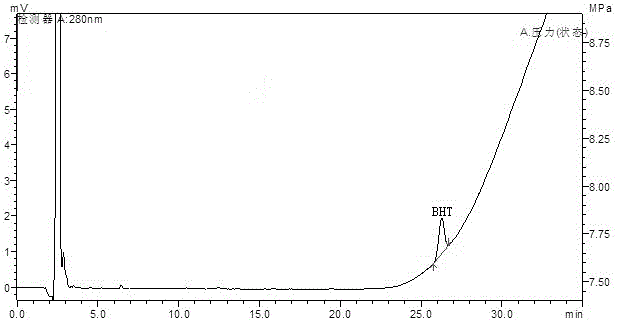
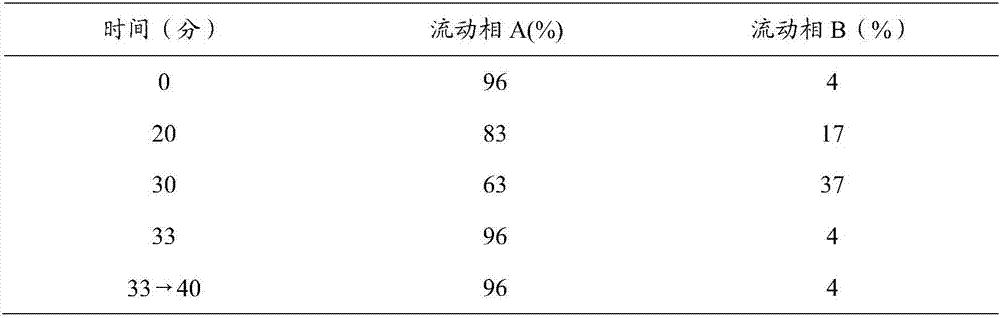
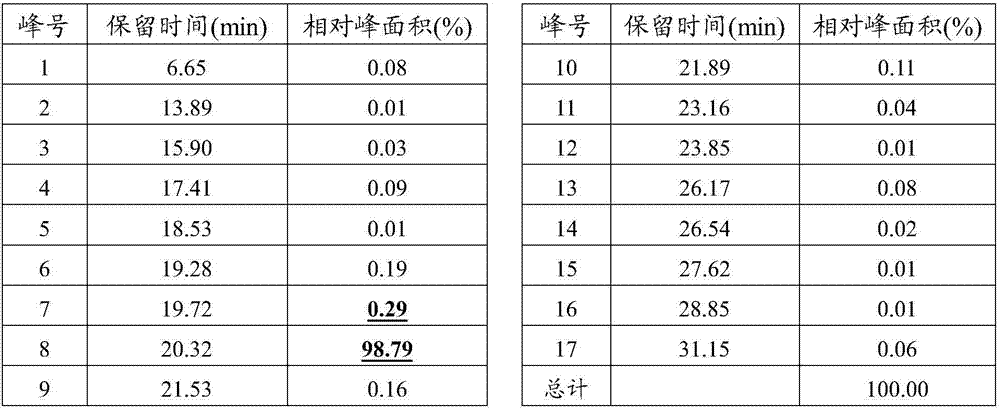
Method for determining antioxidant BHT in insulin glargine injection
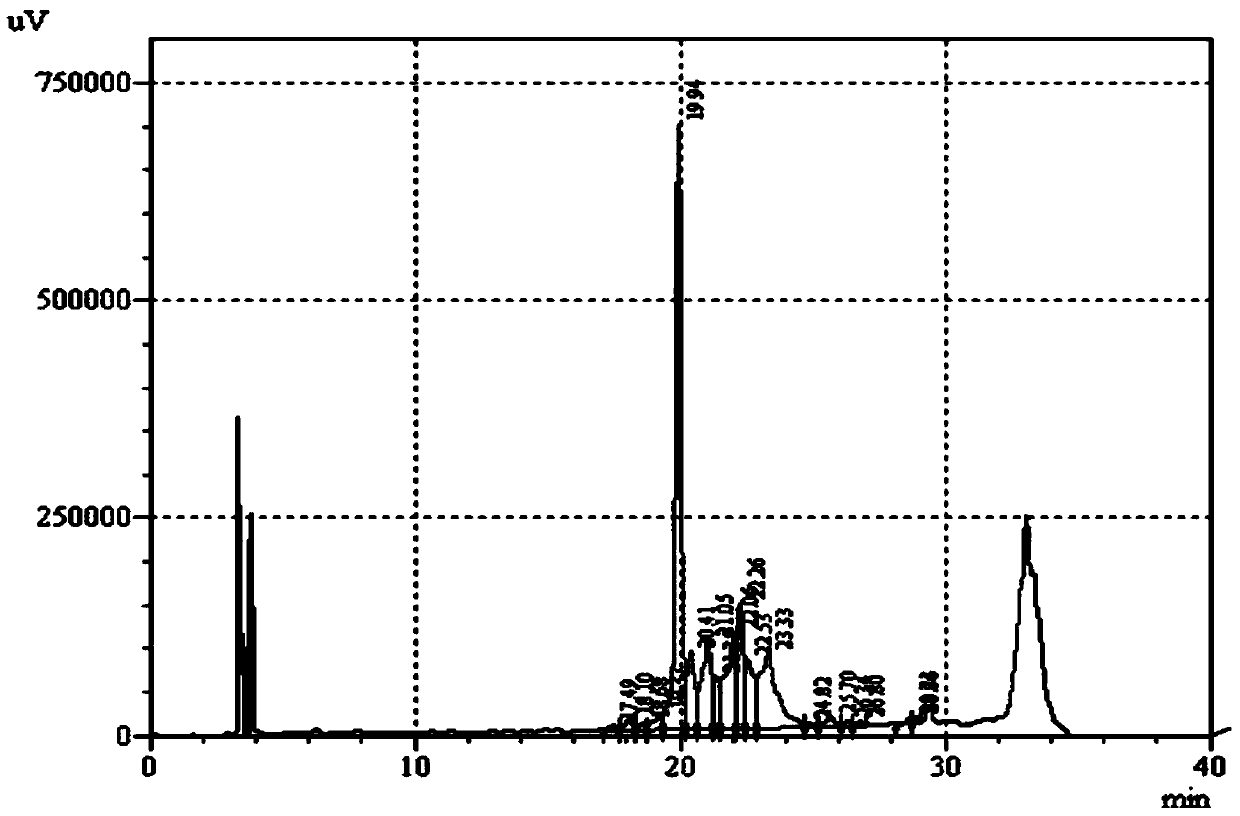
The invention discloses a method for determining antioxidant BHT in an insulin glargine injection and belongs to the field of drug packaging and drug compatibility. The method specifically adopts a HPLC (high performance liquid chromatography) method for detection, a mobile phase comprise acetonitrile, water and a phosphate buffer, a complex preprocessing means is avoided, a chromatographic peak of the antioxidant BHT is separated successfully from chromatographic peaks of insulin glargine and m-cresol according to the method, a trailing phenomenon is avoided, and the content of the antioxidant BHT in a sample is detected accurately.
Owner:广东省医疗器械质量监督检验所
Method for preparing crystalline insulin
ActiveUS20160297862A1Amenable to purifyingLess activityOrganic chemistry methodsPeptide preparation methodsInsulin glargineCrystallization
A method for crystallizing insulin or insulin analogs under alkaline conditions and purifying the insulin or insulin analog crystals by filtering through a filter and drying the insulin or insulin analog crystals captured on the filter to produce crystalline insulin or insulin analog crystal compositions is described. In particular aspects, the method may be used to crystalize insulin glargine.
Owner:MERCK SHARP & DOHME LLC
A kind of preparation method of insulin glargine injection and insulin glargine injection prepared thereof
ActiveCN104688678BQuality improvementProcess impurity reductionPeptide/protein ingredientsMetabolism disorderGlycolurilInsulin injection
The invention provides a preparation method of insulin glargine injection and the prepared insulin glargine injection. The preparation method comprises: dissolving glycerin with part of water for injection, preparing a glycerol solution, and then dividing it into three parts, adding insulin glargine, m-cresol and zinc chloride respectively; Glycerin solution is mixed evenly to obtain mixed solution I; then hydrochloric acid solution is added to the mixed solution I to pH=3.0~3.5, then zinc chloride-glycerin solution is added, stirred evenly, then sodium hydroxide solution is added to pH= 3.5-4.5; finally dilute to the final volume with water for injection. The preparation method of the present invention can rapidly dissolve insulin glargine and other auxiliary materials, significantly shorten the preparation cycle time, reduce process impurities generated during the preparation process, improve the quality of insulin glargine injection, reduce process energy consumption, and improve production efficiency , more suitable for large-scale production needs.
Owner:TONGHUA DONGBAO PHARMA
A kind of extraction method of insulin glargine precursor protein
ActiveCN103833828BHigh protein contentIncrease productivityPeptide preparation methodsCentrifugationInsulin glargine
The invention relates to the field of biochemistry and discloses an extraction method of an insulin glargine precursor protein. The extraction method comprises the following steps of adding an acid into an insulin glargine precursor protein fermentation broth to adjust a pH value to 1-4, carrying out centrifugation and collecting a supernatant so that the insulin glargine precursor protein is obtained. Before bacterium centrifugation, the acid is used to adjust a pH value of the insulin glargine precursor protein fermentation broth to 1-4 so that after extraction, insulin glargine precursor protein content is improved, target protein loss caused by direct centrifugation removal of the bacteria is avoided and an insulin glargine yield is improved.
Owner:YICHANG HEC CHANGJIANG PHARMA CO LTD
A kind of preparation method of insulin glargine and insulin glargine prepared thereof
ActiveCN105585628BSimple componentsLess impuritiesPeptide preparation methodsInsulinsOrganic acidInsulin glargine
The invention provides a preparation method of insulin glargine and insulin glargine prepared by the same. With an insulin glargine solution as a raw material, the method comprises the following steps: mixing the insulin glargine solution, an organic acid, a phenol derivative, a zinc salt and water to obtain crystalline liquid; adjusting pH to 3-4 and preserving heat for 1-8 hours at 25-35 DEG C; adjusting pH to 7.0-8.0 and cooling to 2-8 DEG C; standing for 3-5 hours; centrifuging; and separating the solid and supernate. Compared with prior art, the preparation method provided by the invention realizes higher production efficiency and product quality so as to better guarantee the long-term medication safety of the patients.
Owner:TONGHUA DONGBAO PHARMA
Pharmaceutical composition of insulin glargine and amino acids
There is provided a pharmaceutical composition comprising: (a) insulin glargine, (b) at least two amino acids, and optionally (c) one or more pharmaceutically acceptable excipients. In particular, there is provided a pharmaceutical composition comprising: (a) insulin glargine, (b) arginine and isoleucine in a weight ratio of about 1:2, and optionally (c) one or more pharmaceutically acceptable excipients.
Owner:WOCKHARDT LTD
Method for improving production efficiency of insulin and its analogues
ActiveCN105294854BFacilitates linear scalingHuge industrial application valuePeptide preparation methodsInsulinsEnzyme digestionInsulin glargine
The invention provides a method for improving the efficiency in preparation of insulin and the like. The method comprises the step of conducting chromatography on a precursor of insulin glargine by use of a hydroxyapatite medium. The invention further provides a preparation method for active insulin glargine. The preparation method comprises the step of conducting enzyme digestion on a recombinant expression precursor of insulin glargine by use of a Kex-2p enzyme. In a preferred embodiment of the invention, the preparation method comprises the following steps: obtaining the recombinant expression precursor of insulin glargine; conducting enzyme digestion on the recombinant expression precursor of insulin glargine by use of the Kex-2p enzyme; conducting further chromatographic purification on the enzyme digestion product, so as to obtain active insulin glargine.
Owner:烟台普罗吉医药科技有限公司 +1
A kind of preparation method of insulin glargine crystal
ActiveCN111234001BAccurate analysisHigh diffraction resolutionPeptide preparation methodsInsulinsMedicineCrystal structure
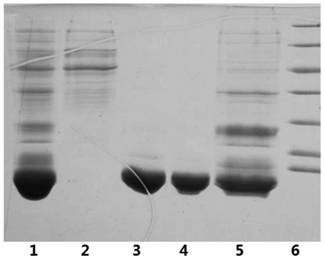
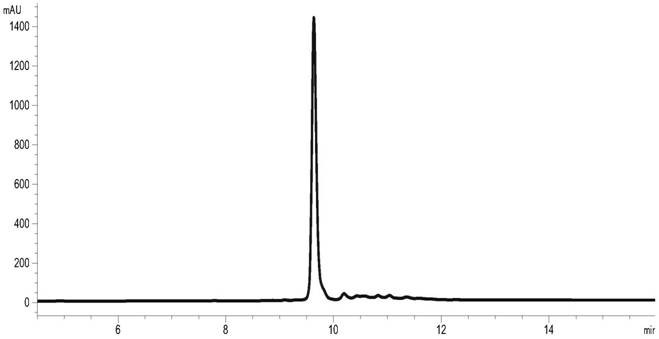
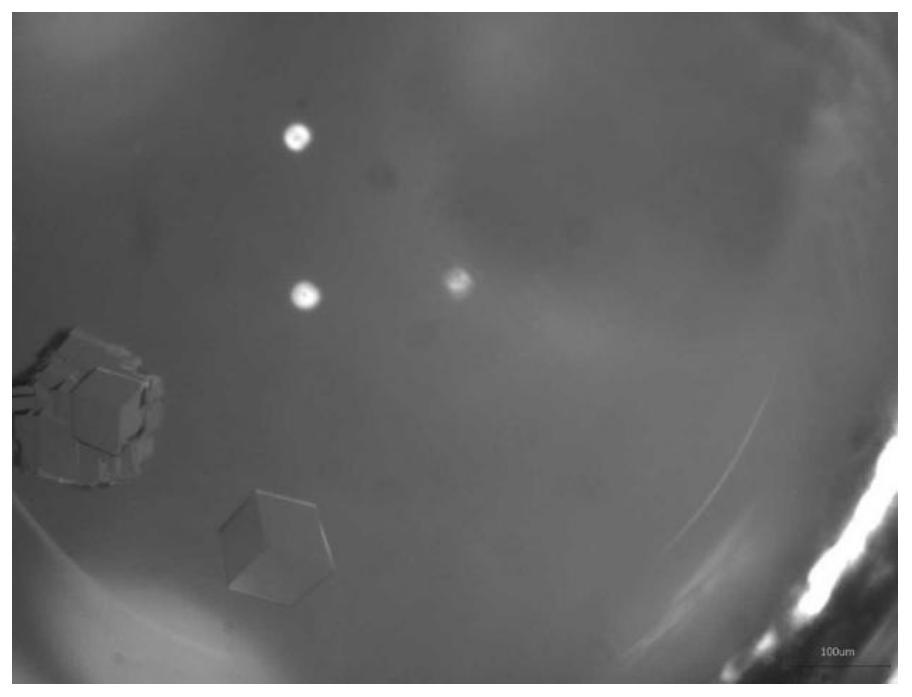
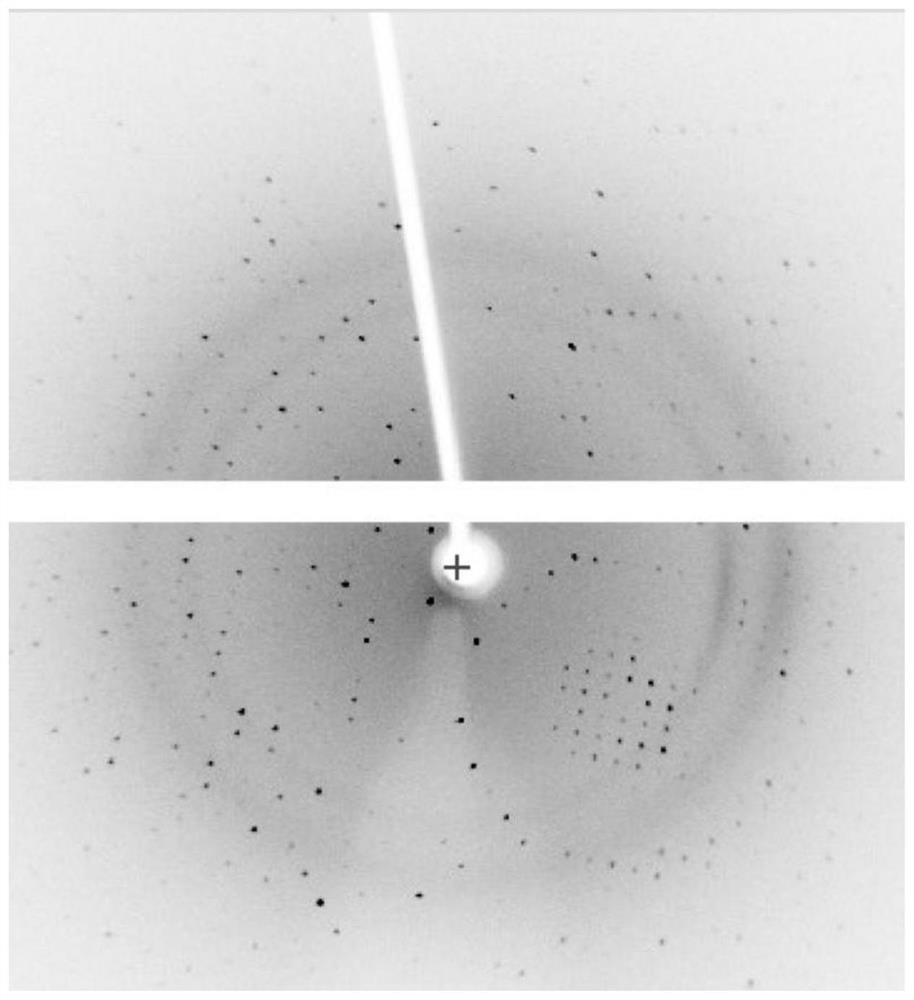
The invention relates to the technical field of insulin crystallization, in particular to a method for preparing insulin glargine crystals. The method for preparing insulin glargine crystals adopts the gas-phase diffusion hanging drop method, comprising the following steps: (1) preparing a crystallization solution containing the following components: Tris 0.2-1.0M, citric acid 0.2-1.0M; the pH of the crystallization solution is 8.0 to 11.0; (2) Mix the insulin glargine solution with the crystallization solution to obtain a mixed crystallization solution; (3) Hang the mixed crystallization solution above the mother liquor pool filled with the crystallization solution, and let it stand for cultivation . The method can be used to obtain insulin glargine crystals with regular shape and suitable size in the form of single crystals, and the resolution of X-ray diffraction is high, and the crystal structure of insulin glargine can be correctly analyzed.
Owner:YICHANG HEC CHANGJIANG PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com