Method for improving efficiency in preparation of insulin and like
A technology of insulin glargine and its precursor, which is applied in the field of biopharmaceuticals and can solve problems such as limiting the scope of application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Embodiment 1: recombinant insulin glargine fusion protein expression
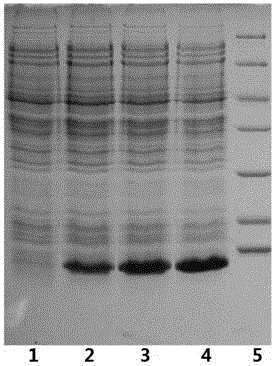
[0049] Referring to Novagen's "pET System Operation Manual" (10th edition), the insulin glargine fusion protein plasmid was constructed according to conventional molecular cloning techniques, and then transferred into the pET30aBL-21 (DE3) Escherichia coli expression strain to obtain the insulin glargine fusion protein engineering bacteria. Fermentation obtains recombinant expressed glargine fusion protein, under 100L fermentation volume, OD 600 reached 150, the target protein expression rate can reach 42% ( figure 1 ).
Embodiment 2
[0050] Embodiment 2: Purification of denatured protein of insulin glargine fusion protein
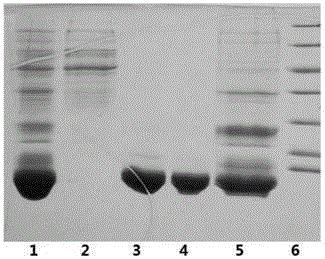
[0051] The insulin glargine fusion protein fermentation broth obtained according to the method of Example 1 was centrifuged (9000rpmx20min), the collected bacteria were crushed three times with a high-pressure homogenizer, and the precipitate was collected by centrifugation (9000rpmx30min). The pellet was resuspended with a resuspension buffer containing 20mM Tris, 2M urea, 5mM EDTA, 10mM dithiothreitol, and 1% Triton-100, homogenized again three times with a high-pressure homogenizer, and collected by centrifugation (13000rpmx30min). The obtained inclusion bodies were dissolved with 8M urea, and 20mM dithiothreitol was added.
[0052] Using hydroxyapatite medium (such as CHT TM CeramicHydroxyapatite) was subjected to chromatographic purification of the denatured protein obtained by the above method. Refer to Bio-Rad's "CHT TM CeramicHydroxyapatiteInstructionManual "page 13 method, a...
Embodiment 3
[0053] Embodiment 3: Refolding of insulin glargine fusion protein
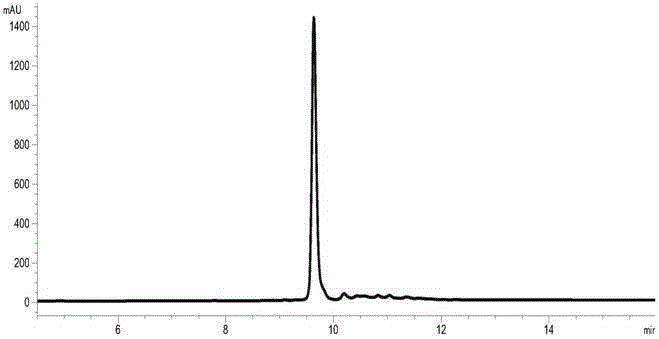
[0054] Dilute the insulin glargine fusion protein denatured protein solution obtained according to the method of Example 2 into the refolding buffer to obtain the insulin glargine fusion protein refolding protein, and the refolding buffer contains 20mMNaH 2 PO 4 , 20mM NaAc, 5mMGSH, 1mMGSSG, the pH value is 5.0. When the concentration of the target protein is 10mg / ml, the renaturation rate is 91%. The obtained insulin glargine fusion protein refolding protein solution is concentrated and subjected to buffer replacement, and the replacement buffer contains 20mMNaH 2 PO 4 , 20mM Tris, pH8.0. The results of RP-HPLC detection of renaturation rate are shown in image 3 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com