Method for improving production efficiency of insulin and its analogues
A technology of insulin glargine and its precursor, which is applied in the field of biopharmaceuticals and can solve problems such as limiting the scope of application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
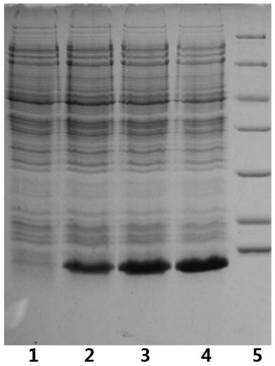
[0048] Embodiment 1: recombinant insulin glargine fusion protein expression
[0049] Referring to Novagen's "pET System Operation Manual" (10th edition), the insulin glargine fusion protein plasmid was constructed according to conventional molecular cloning techniques, and transferred into pET30a BL-21(DE3) Escherichia coli expression strain to obtain the insulin glargine fusion protein engineering bacteria , fermented recombinantly expressed insulin glargine fusion protein, under 100L fermentation volume, OD 600 reached 150, the target protein expression rate can reach 42% ( figure 1 ).
Embodiment 2
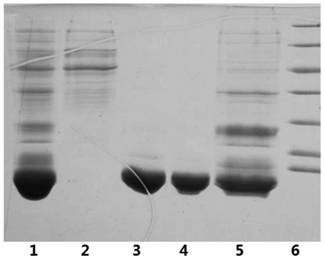
[0050] Embodiment 2: Purification of denatured protein of insulin glargine fusion protein
[0051] The insulin glargine fusion protein fermentation liquid obtained according to the method of Example 1 was centrifuged (9000rpm x 20min), the collected bacteria were crushed three times with a high-pressure homogenizer, and the precipitate was collected by centrifugation (9000rpm x 30min). Resuspend the pellet with a resuspension buffer containing 20mM Tris, 2M urea, 5mM EDTA, 10mM dithiothreitol, and 1% Triton-100, homogenize again three times with a high-pressure homogenizer, and collect the pellet by centrifugation (13000rpm x 30min) . The obtained inclusion bodies were dissolved with 8M urea, and 20mM dithiothreitol was added.
[0052] Using hydroxyapatite medium (such as CHT TM Ceramic Hydroxyapatite) carries out chromatographic purification to the denatured protein obtained by the above-mentioned method. Refer to Bio-Rad's "CHT TMCeramic Hydroxyapatite InstructionManual...
Embodiment 3
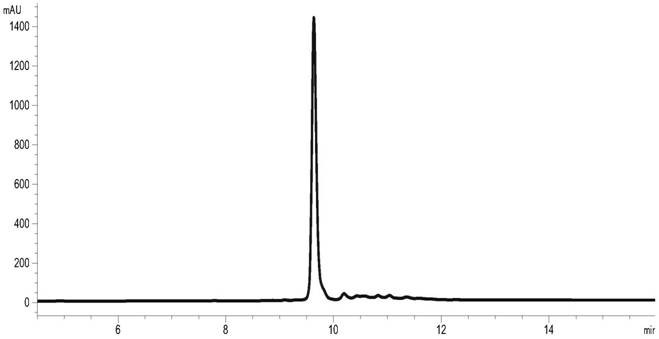
[0053] Embodiment 3: Refolding of insulin glargine fusion protein
[0054] Dilute the insulin glargine fusion protein denatured protein solution obtained according to the method of Example 2 into the refolding buffer to obtain the insulin glargine fusion protein refolding protein, and the refolding buffer contains 20mM NaH 2 PO 4 , 20mM NaAc, 5mM GSH, 1mM GSSG, the pH value is 5.0. When the concentration of the target protein is 10mg / ml, the renaturation rate is 91%. Concentrate the obtained insulin glargine fusion protein refolding protein solution and perform buffer replacement, the replacement buffer contains 20mM NaH 2 PO 4 , 20mM Tris, pH 8.0. The results of RP-HPLC detection of renaturation rate are shown in image 3 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com