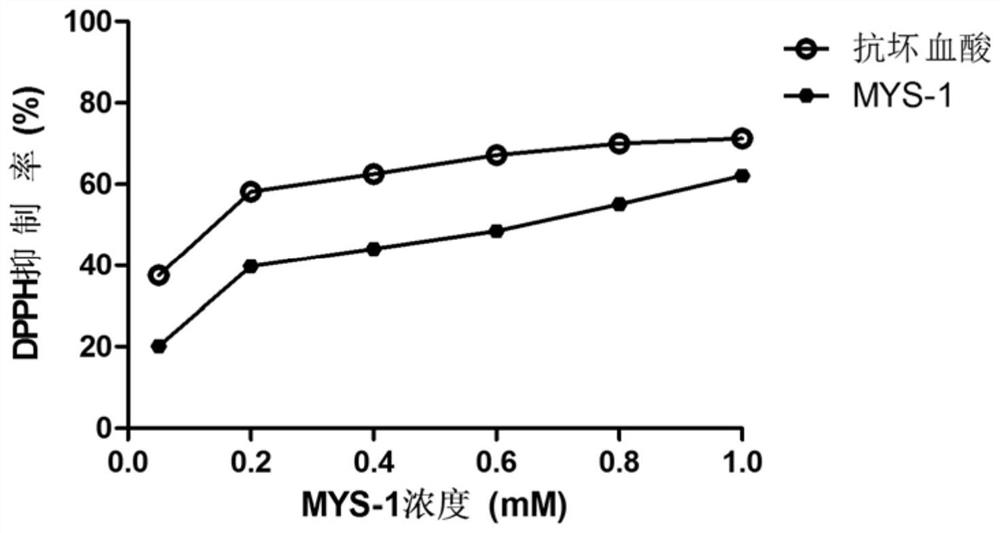
A kind of gene recombinant collagen oligopeptide mys-1 and its preparation method and application
A technology of MYS-1 and collagen oligopeptide, applied in the field of genetic engineering, can solve the problem of high cost of collagen, achieve better biological safety, small molecular weight, and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Construction and Expression of Expression Engineering Bacteria pET32a-MYS-1 / BL21(DE3)
[0061] Specific steps are as follows:
[0062] (1) Synthesis of MYS-1 gene:
[0063] Primer design:
[0064] Primer 1:
[0065]
[0066] Primer 2:
[0067]
[0068] GGTACC is the KpnI restriction site, CTCGAG It is the XhoI restriction site.
[0069] The amount of each component of annealing: (primer concentration is 1OD dissolved in 400μL ddH 2 O, reaction system: 20μL)
[0070] Primer 1: 2 μL, Primer 2: 2 μL, T4 PNK 1 μL (10 U), ATP 20 mM, ddH 2 O replenish water to 20 μL;
[0071] The procedure for synthesizing the MYS-1 gene: 95°C, 5min; 95°C, 1min, 56°C, 1min, 72°C, 1min, 30 cycles; 72°C, 10min;
[0072] Cool naturally to room temperature to obtain the product for later use.
[0073] (2) Construction of recombinant vector pET32a-MYS-1:
[0074] Use KpnI enzyme and XhoI enzyme to perform double enzyme digestion on the plasmid pET32a (purchased from Shanghai Sa...
Embodiment 2
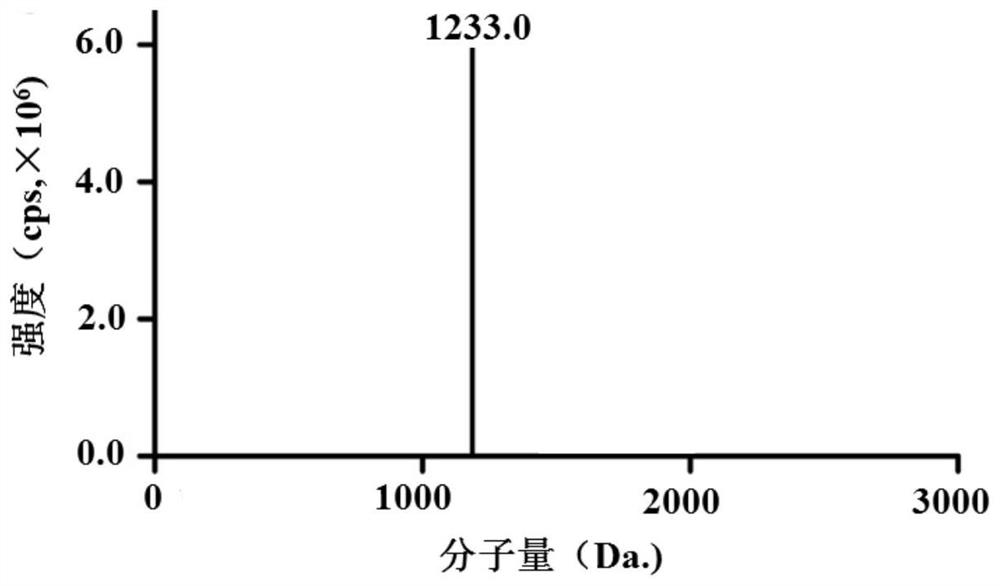
[0080] Purification, preparation and identification of recombinant human collagen oligopeptide MYS-1
[0081] Pipette and mix 2mL of medium into the column (Ni-NTA column, GenScript Biotechnology Co., Ltd., 10mL column volume), add 4 times the column volume of Lysis equilibration buffer to equilibrate the chromatography medium; The supernatant was loaded onto the column at a flow rate of 1.0mL / min; with 8 times the column volume of washing buffer (50mM Na 2 HPO 4 , 0.3M NaCl, 10~50mM imidazole, pH=8.0) to pass through the column at 1.0mL / min to wash away the impurity protein or fusion protein not bound by affinity, and use 10 times column volume of elution buffer at 1.0mL / min Min passed through the column, collected the eluate, took the sample effluent, washing effluent, and eluted effluent for SDS-PAGE electrophoresis to detect the purification effect of the fusion protein Trx-6His-MYS-1. The fusion protein with most of the foreign proteins removed was obtained in the solut...
Embodiment 3
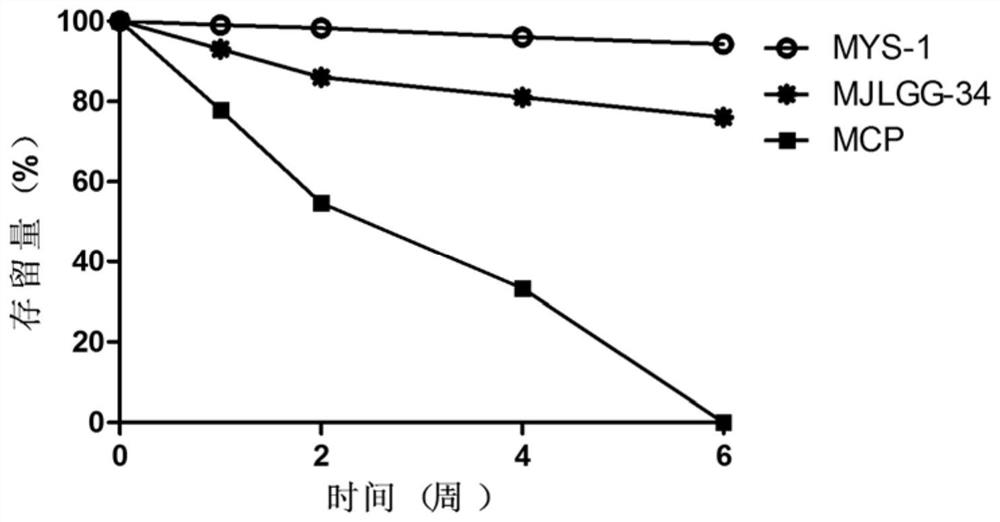
[0090] Stability detection of recombinant human collagen oligopeptide MYS-1
[0091] Recombinant human collagen oligopeptide MYS-1 was dissolved in 20mM sodium phosphate buffer (containing 150mM sodium chloride) (pH 8.0) at a concentration of 1mg / mL, incubated at 37°C, and analyzed using a liquid chromatography-mass spectrometry system (LC-MS) detects the stock flow of recombinant human collagen oligopeptide MYS-1 in the solution over time (1 to 6 weeks), calculates the depletion rate of MYS-1 over time, and uses marine collagen peptide (MCP) and recombinant collagen Peptide MJLGG-34 was used as a control. The genetic recombinant collagen-like peptide MJLGG-34 is disclosed in "201811505180.3, Genetic recombinant collagen-like peptide MJLGG-34 and its preparation method and application".
[0092] Such as figure 2As shown, when incubated for 1 week, MCP was reduced by 22.1%, and when incubated for 4 weeks, MCP was reduced by 66.7%; 18.9% and 24.0%; while the gene recombinant...
PUM
| Property | Measurement | Unit |
|---|---|---|
| clearance rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com