Insulin glargine injection and preparation method thereof
A technology for insulin glargine and injection, applied in the field of medicine, can solve the problems of high production cost, white spots in insulin glargine injection, and no mention of the stability of insulin preparations, etc., and achieve the effect of stable quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
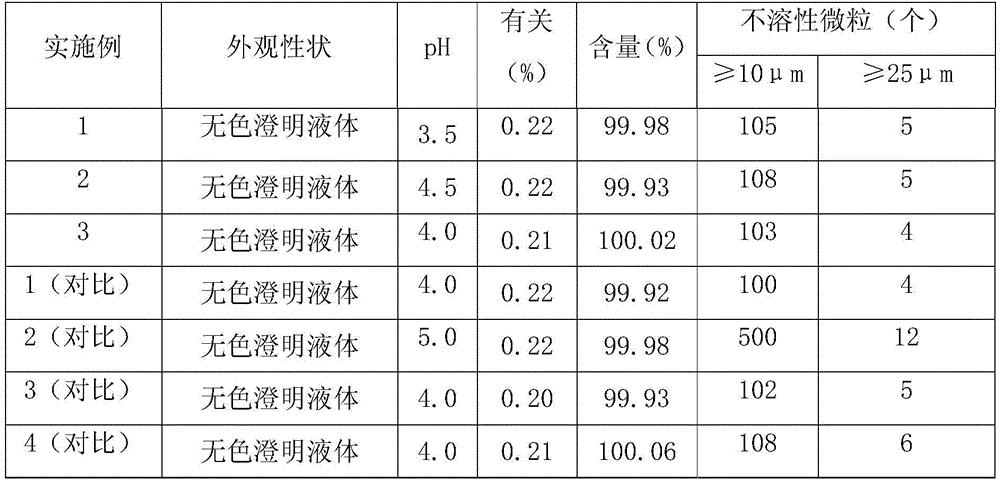
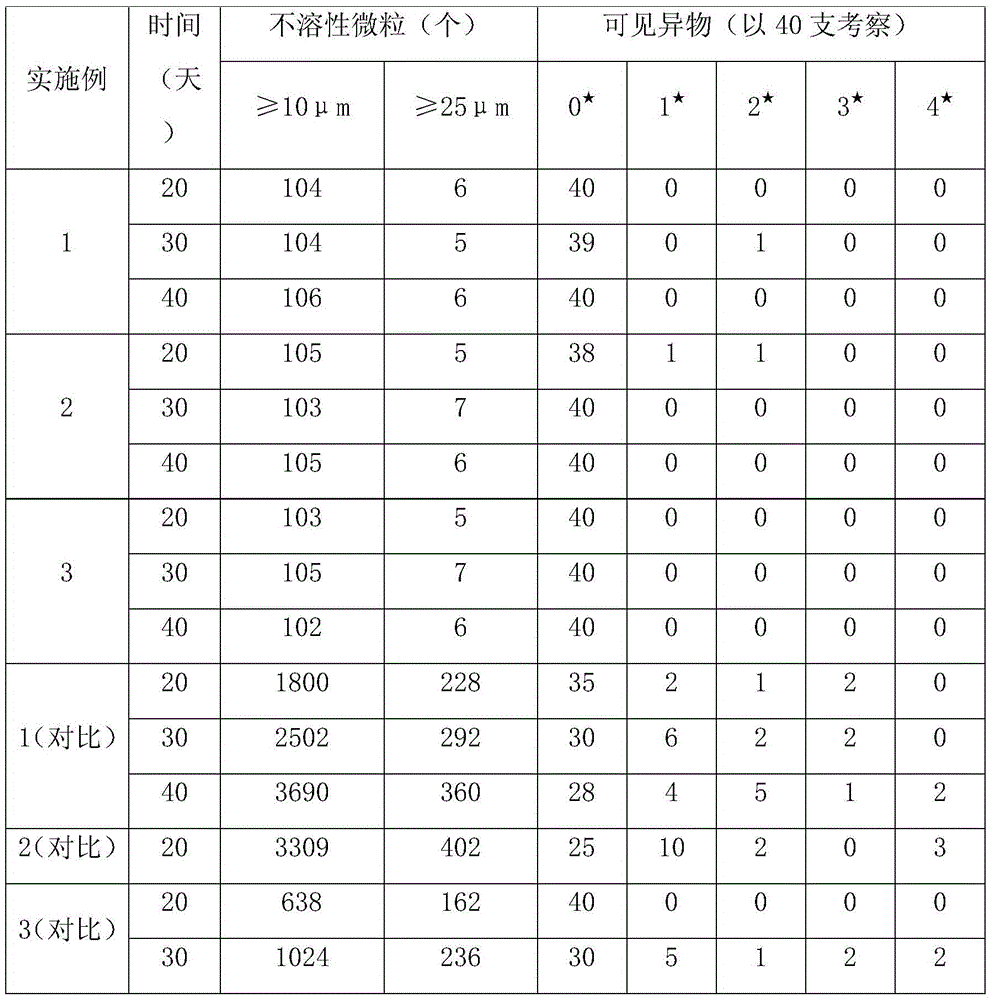
[0023] Add 1500ml of water for injection pre-cooled to room temperature in the beaker, add 7.27g of insulin glargine of the prescribed amount, add 1M hydrochloric acid solution while stirring to make it dissolve, add m-cresol 5.4g of the prescribed amount, 0.375g of zinc chloride, Benzalkonium chloride 5.5g, stir and mix, adjust the pH value of the solution to 3.5, add 85% glycerin 40.0g, absolute ethanol 40.0g, set the volume to 2000ml, stir the solution to a colorless clear liquid; filter, fill, Light inspection is the finished product.
Embodiment 2
[0025] Add 1500ml of water for injection pre-cooled to room temperature in the beaker, add 7.27g of insulin glargine of the prescribed amount, add 1M hydrochloric acid solution while stirring to make it dissolve, add m-cresol 5.4g of the prescribed amount, 0.375g of zinc chloride, Benzalkonium chloride 6.5g, stir and mix, adjust the pH value of the solution to 4.5, add 85% glycerin 40.0g, absolute ethanol 50.0g, set the volume to 2000ml, stir the solution to a colorless clear liquid; filter, fill, Light inspection is the finished product.
Embodiment 3
[0027] Add 1500ml of water for injection pre-cooled to room temperature in the beaker, add 7.27g of insulin glargine of the prescribed amount, add 1M hydrochloric acid solution while stirring to make it dissolve, add m-cresol 5.4g of the prescribed amount, 0.375g of zinc chloride, Benzalkonium chloride 6.0g, stir and mix, adjust the pH value of the solution to 4.0, add 85% glycerin 40.0g, absolute ethanol 45.0g, set the volume to 2000ml, stir the solution to a colorless clear liquid; filter, fill, Light inspection is the finished product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com