Preparation method of insulin glargine injection and insulin glargine injection prepared by using preparation method
A technology for insulin glargine and injection, which is applied in the field of insulin glargine injection, can solve the problems of lowering the stability of insulin glargine, achieve the effects of reducing the probability of adverse reactions, shortening the preparation time, and reducing process impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
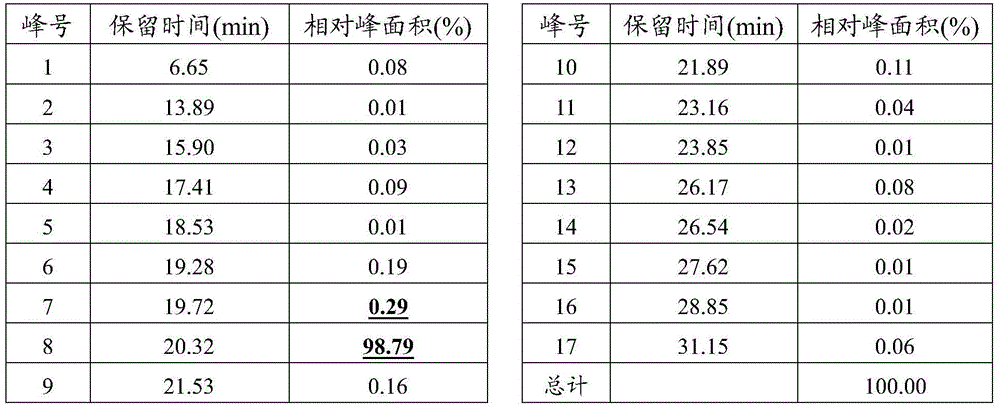
Examples
preparation example Construction
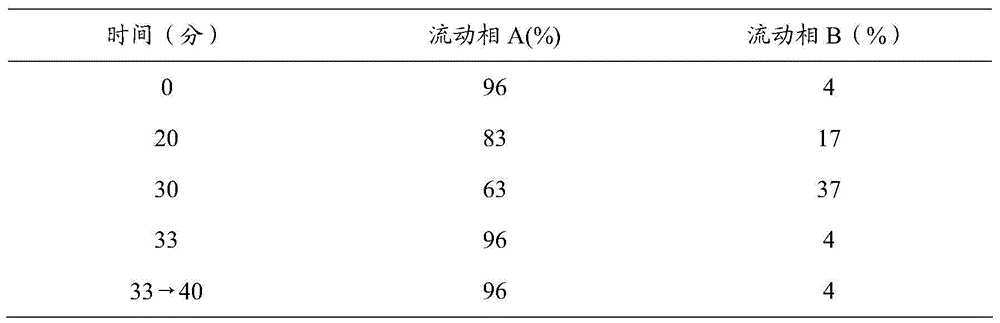
[0041] Preparation method 1 (preparation method provided by the present invention): (1) take a 1L beaker, add a rotor, place it on a magnetic stirrer, add glycerin and 600mL water for injection, and dissolve the glycerin under stirring to obtain a glycerin solution; (2) Pre-dissolve the glycerol solution described in 180mL, 300mL, and 120mL of refined insulin, m-cresol, and zinc chloride respectively to obtain insulin glargine-glycerol solution, m-cresol-glycerol solution and zinc chloride-glycerol solution; (3) the The insulin glargine-glycerin solution is mixed with the m-cresol-glycerin solution, and stirred evenly to obtain a mixed solution I; (4) under stirring, add 0.1mol / L hydrochloric acid solution to the mixed solution I until pH=3.0~ 3.5, insulin glargine dissolves rapidly, and the solution is clear; (5) Add zinc chloride-glycerin solution, stir evenly; (6) Dilute to 900mL with water for injection, add 0.1mol / L sodium hydroxide solution to adjust pH=4.0 (7) Dilute to...
Embodiment 1
[0046] Example 1 Insulin glargine injection prepared by different methods
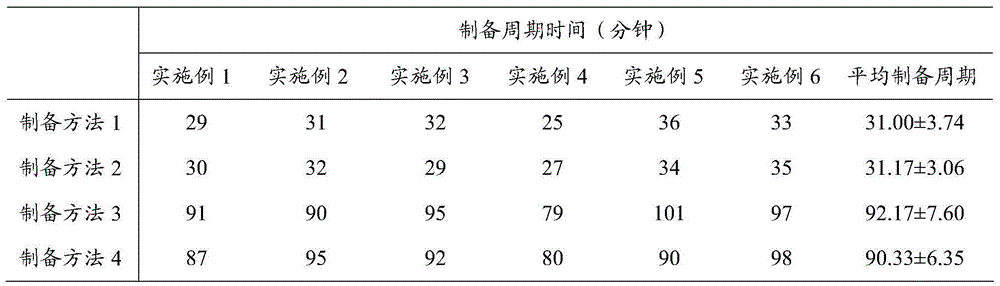
[0047] The insulin glargine injection described in this implementation contains insulin glargine 100IU / mL, glycerin 17mg / mL, m-cresol 2.7mg / mL, zinc 30μg / mL, hydrochloric acid, sodium hydroxide and water for injection, pH=4; according to the above Preparation method 1, preparation method 2, preparation method 3 and preparation method 4 prepared insulin glargine injection respectively, and recorded the time required for the preparation cycle. The results are shown in Table 1.
Embodiment 2
[0048] Example 2 Insulin glargine injection prepared by different methods
[0049] The insulin glargine injection described in this embodiment contains insulin glargine 100 IU / mL, glycerin 16 mg / mL, m-cresol 3.2 mg / mL, zinc 27 μg / mL, hydrochloric acid, sodium hydroxide and water for injection, pH=4. Insulin glargine injection was prepared respectively according to the above-mentioned preparation method 1, preparation method 2, preparation method 3 and preparation method 4, and the time required for the preparation cycle was recorded. The results are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com