Insulin glargine derivative and application thereof
A technology for insulin glargine and arginine, which is applied in the field of insulin glargine derivatives, and can solve problems such as low yield, high production cost, and complicated insulin glargine process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0188] Construction and expression of embodiment 1 insulin glargine expression strain
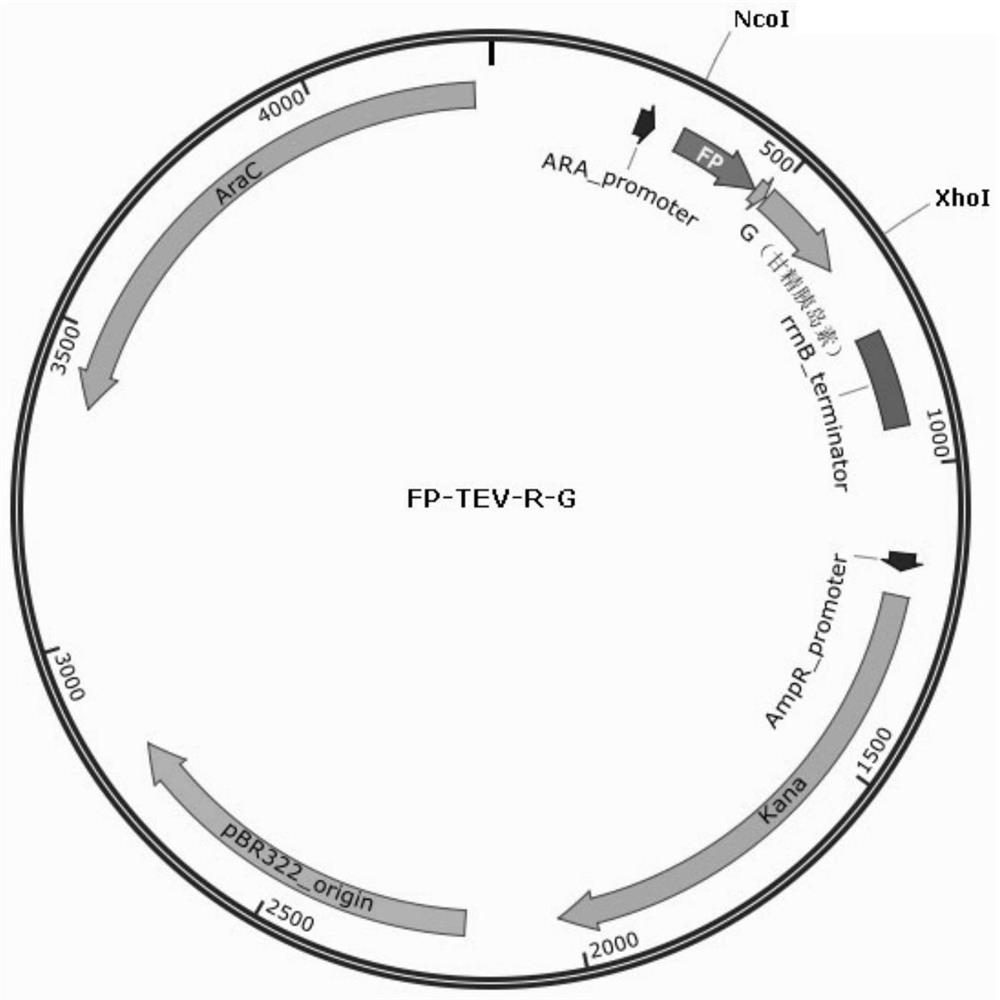
[0189] To construct the insulin glargine expression plasmid, the construction method refers to the existing technology in the field, specifically, refer to the description in the examples in the patent application number 201910210102.9. The DNA fragment of the fusion protein FP-TEV-R-G was cloned into the NcoI-XhoI site downstream of the araBAD promoter of the expression vector plasmid pBAD / His A (purchased from NTCC, kanamycin resistance) to obtain the plasmid pBAD-FP -TEV-R-G. Plasmid map such as figure 1 shown.
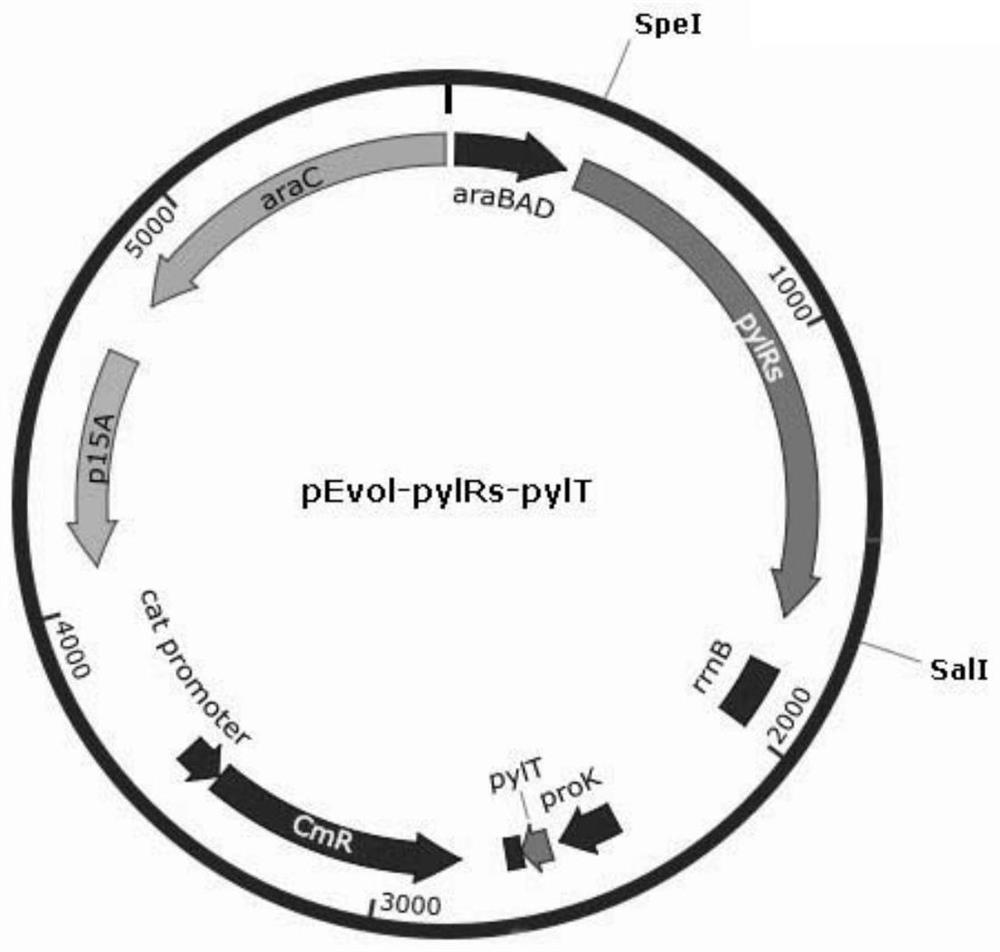
[0190] Then the DNA sequence of pylRs was cloned into the SpeI-SalI site downstream of the araBAD promoter of the expression vector plasmid pEvol-pBpF (purchased from NTCC Company, chloramphenicol resistance), and at the same time, the pylRs was inserted downstream of the proK promoter by PCR. DNA sequence of tRNA (pylTcua) of aminoacyl-tRNA synthetase. This plasmid was nam...
Embodiment 2
[0203]Example 2 Dissolution and renaturation of inclusion bodies
[0204] Add 8mol / L urea solution to the obtained inclusion body, adjust the pH to 8.0-9.0 with sodium hydroxide, stir at room temperature for 1-3h, control the protein concentration to 10-20mg / mL, and add β-mercaptoethanol to a final concentration of 10-20mmol / L, continue stirring for 0.5-1.0h.
[0205] Add the inclusion body solution dropwise to the renaturation buffer, dilute 5-10 times for renaturation, maintain the pH of the renaturation solution at 9.0-10.5, temperature 2-8°C, and stir for 10-20 hours for renaturation.
Embodiment 3
[0206] Embodiment 3 Fusion proteolysis
[0207] Use 10KD ultrafiltration membrane to concentrate the refolding solution 8-10 times. Add dilute hydrochloric acid to the refolding solution to adjust the pH to 7.5-9.0. The protein concentration of the refolding concentrate was determined by the Bradford method, and the total protein amount was calculated. Add recombinant trypsin under stirring, the ratio of recombinant trypsin to the total protein of refolding solution is 1:3000~1:10000, add 30mmol / L succinic acid or 30mmol / L L-lysine, and the enzymatic digestion temperature is 15- At 25°C, the digestion time is 14-20 hours.
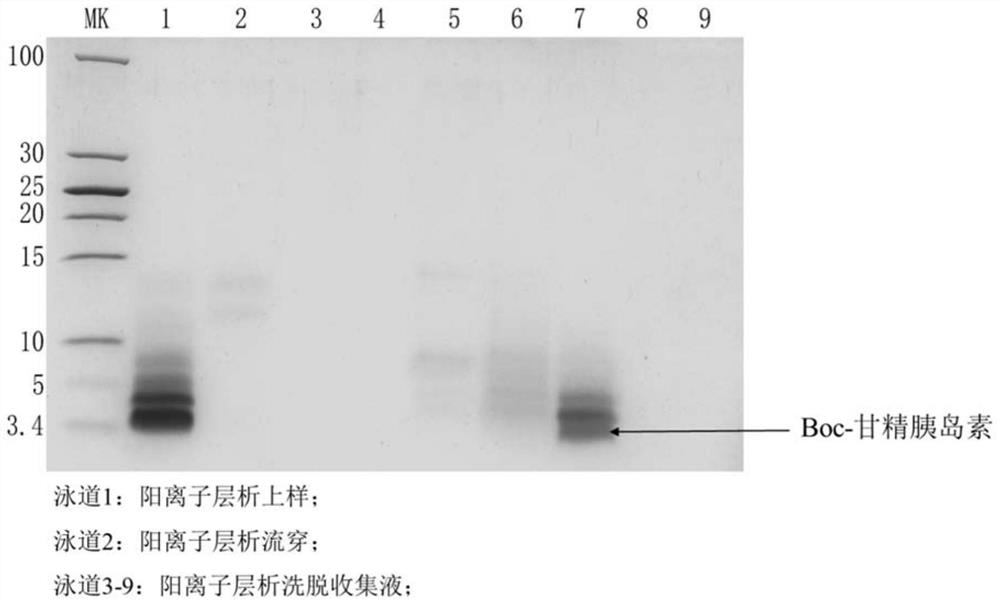
[0208] After 10 hours of enzyme digestion, the content of Boc-insulin glargine in the enzyme digestion solution was detected by HPLC. When the difference between the concentrations of Boc-insulin glargine detected for two consecutive hours was less than 3%, the enzyme digestion was completed. Finally, the concentration of Boc-insulin glargine in the dige...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com