Renaturation method of insulin glargine precursor
A technology of insulin glargine and precursor, applied in the preparation methods, chemical instruments and methods of insulin and peptides, etc., can solve problems such as low recovery rate, achieve the effects of less by-products, shorten reaction time, and increase protein content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Refolding of embodiment 1 insulin glargine precursor
[0057] At room temperature, dissolve 10 grams of insulin glargine precursor in 588 mL of 6M, pH9.5 urea solution to make the protein concentration of insulin glargine precursor about 17 g / L; then add 5 g of DTT to adjust the pH value to 9.5, control The temperature of the reaction system was 35° C., and the reaction was carried out for 30 minutes.
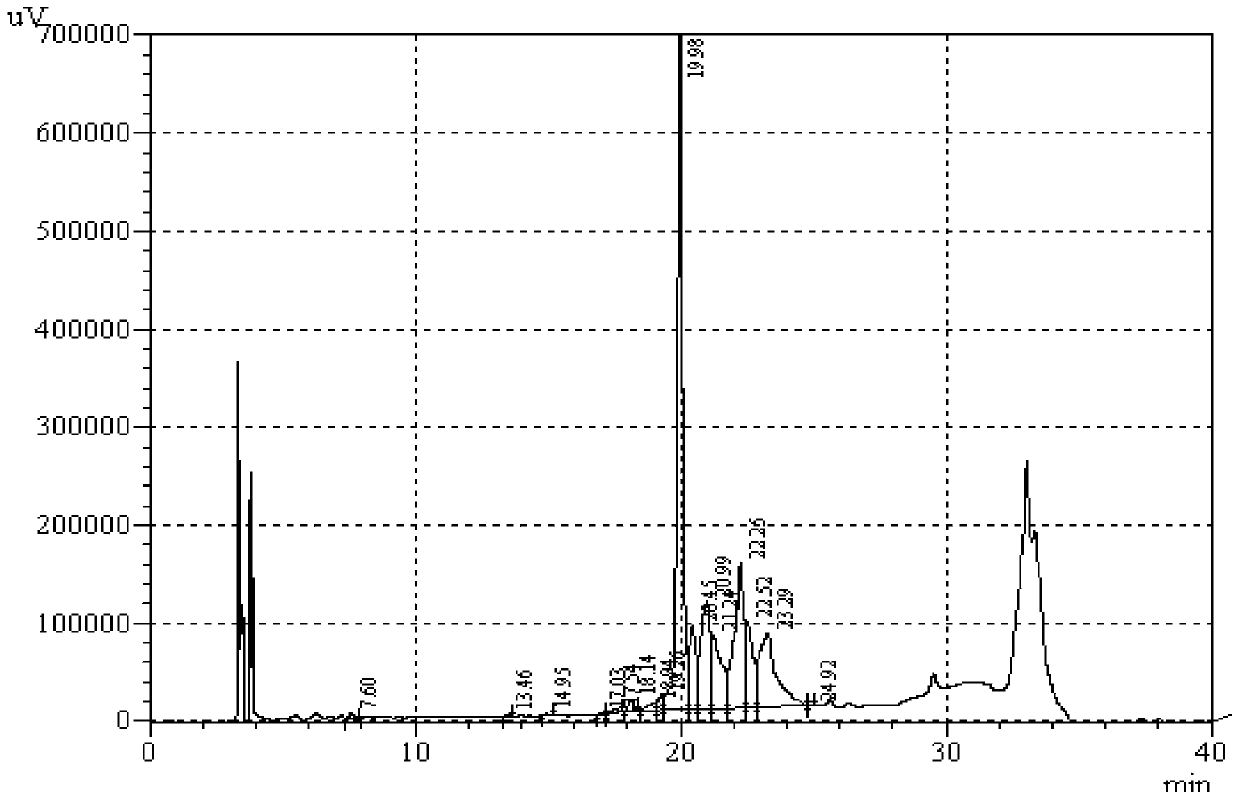
[0058] Add the denatured insulin glargine precursor solution to 10 mM, pH9.5 Tris-HCl buffer solution, and use the Tris-HCl buffer solution to set the volume to 10 L, so that the protein concentration of the insulin glargine precursor is 1.0 g / L, Then add 5g PEG4000, 500mL glycerol, 8mg CuSO 4 , adjust the pH value to 9.5, with 20cm 3 Air was continuously fed into the refolding solution at a rate of / H, the temperature of the reaction system was controlled at 4°C, and the reaction was stopped after 12 hours of reaction. Take 20 μL of the refolding solution and analyze...
Embodiment 2
[0059] Refolding of embodiment 2 insulin glargine precursor
[0060] At room temperature, dissolve 10 grams of insulin glargine precursor in 357 mL of 8M, pH 11.5 urea solution, so that the protein concentration of insulin glargine precursor is about 28 g / L, add 2.5 g of DTT, adjust the pH value to 11.5, control The temperature of the reaction system was 45° C., and the reaction was carried out for 60 minutes.
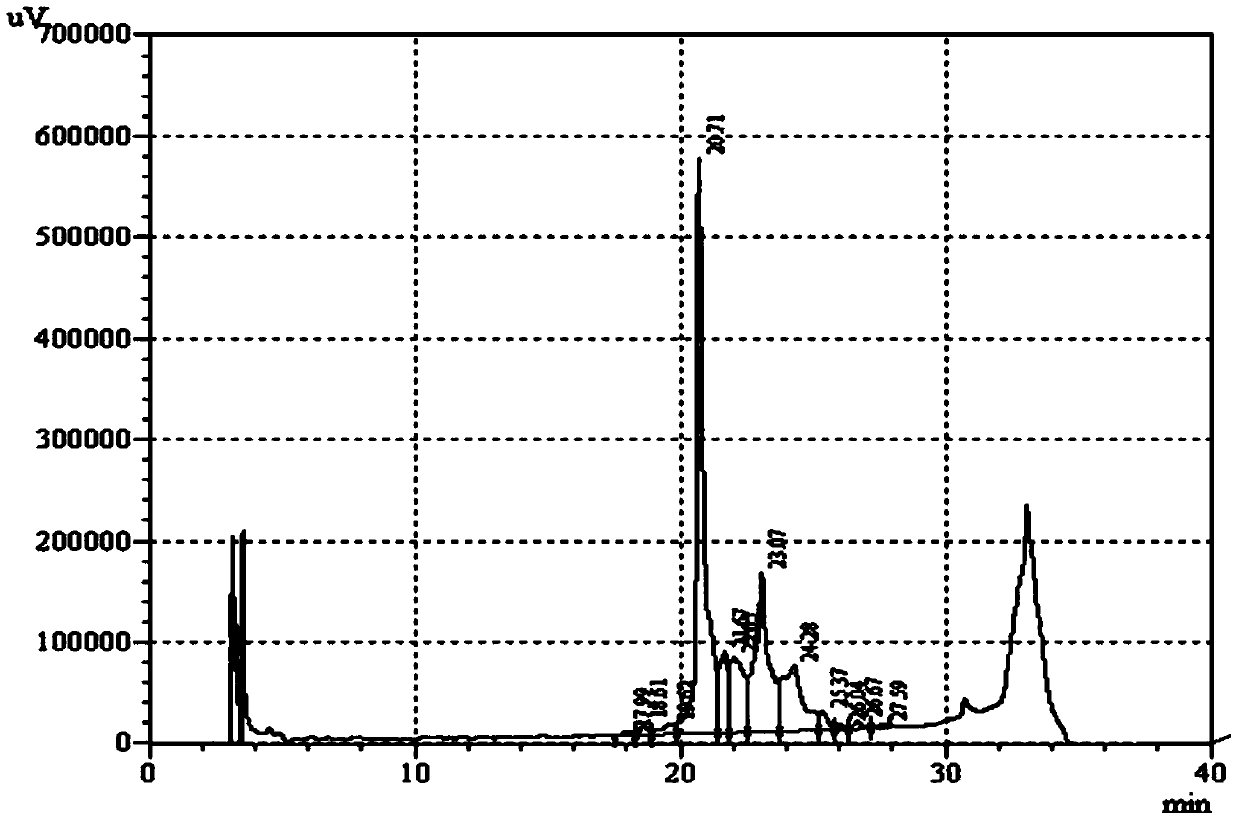
[0061] Add the denatured insulin glargine precursor solution to 50mM, pH11.5 Tris-HCl buffer solution, and use the Tris-HCl buffer solution to set the volume to 7.14L, so that the protein concentration of the insulin glargine precursor is 1.4g / L , then add 14.28g PEG4000, 1428mL glycerol, 22.85mg CuSO 4 , adjust the pH value to 11.5, to 714cm 3 Air was continuously fed into the refolding solution at a rate of / H, the temperature of the reaction system was controlled at 15°C, and the reaction was stopped after 25 hours of reaction. Take 20 μL of the refolding solutio...
Embodiment 3
[0062] Refolding of embodiment 3 insulin glargine precursor
[0063] At room temperature, dissolve 10 grams of insulin glargine precursor in 588 mL of 6M, pH 9.5 urea solution to make the protein concentration of insulin glargine precursor about 17 g / L, add 5 g of cysteine hydrochloride to adjust the pH The value is 9.5, the temperature of the reaction system is controlled at 35° C., and the reaction is carried out for 30 minutes.
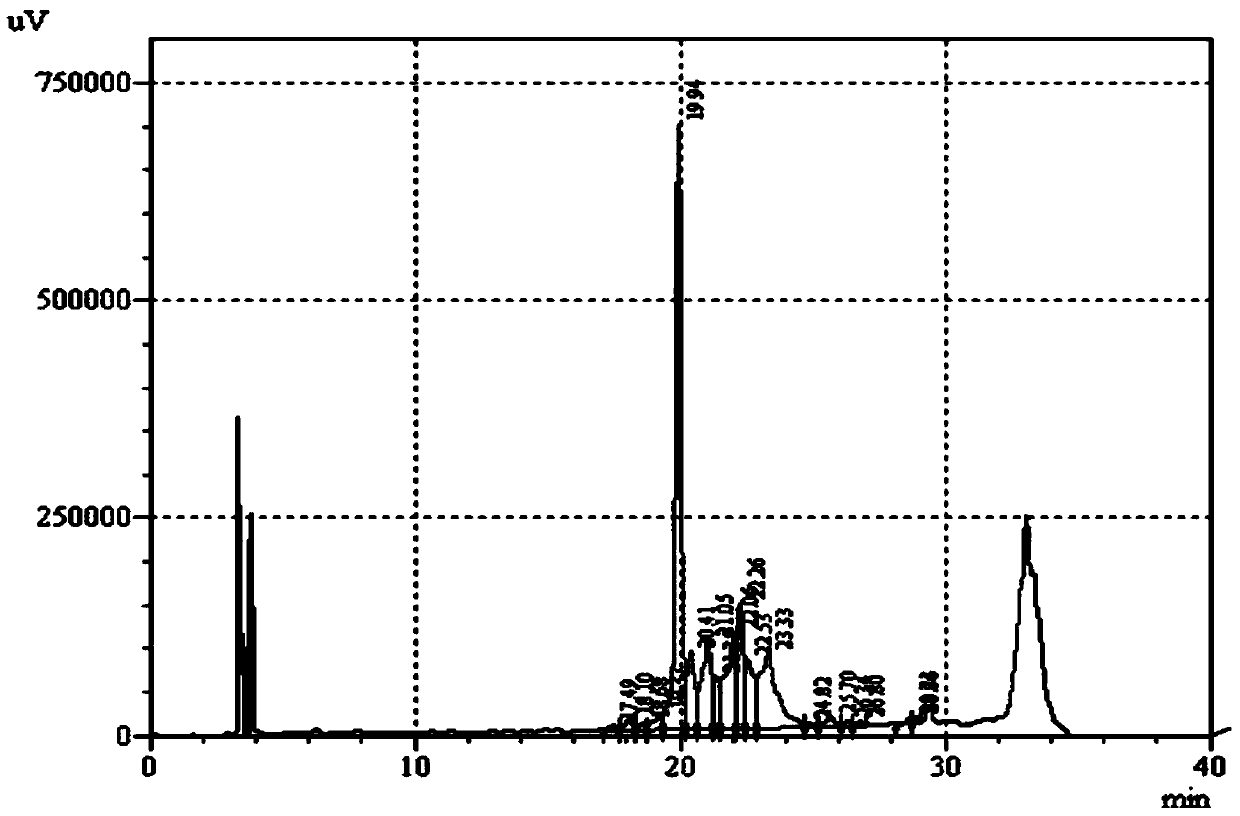
[0064] Add the denatured insulin glargine precursor solution to 10mM, pH9.5 glycine dilution buffer, and dilute to 10L with the glycine dilution buffer to make the protein concentration of the insulin glargine precursor 1.0g / L, then add 5g PEG4000, 500mL glycerol, 6.75mg CuCl 2 , adjust the pH value to 9.5, with 20cm 3 Air was continuously fed into the refolding solution at a rate of / H, and the temperature of the reaction system was controlled at 4 ° C. After 12 hours of reaction, the reaction was stopped. Take 20 μL of the refolding solution...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com