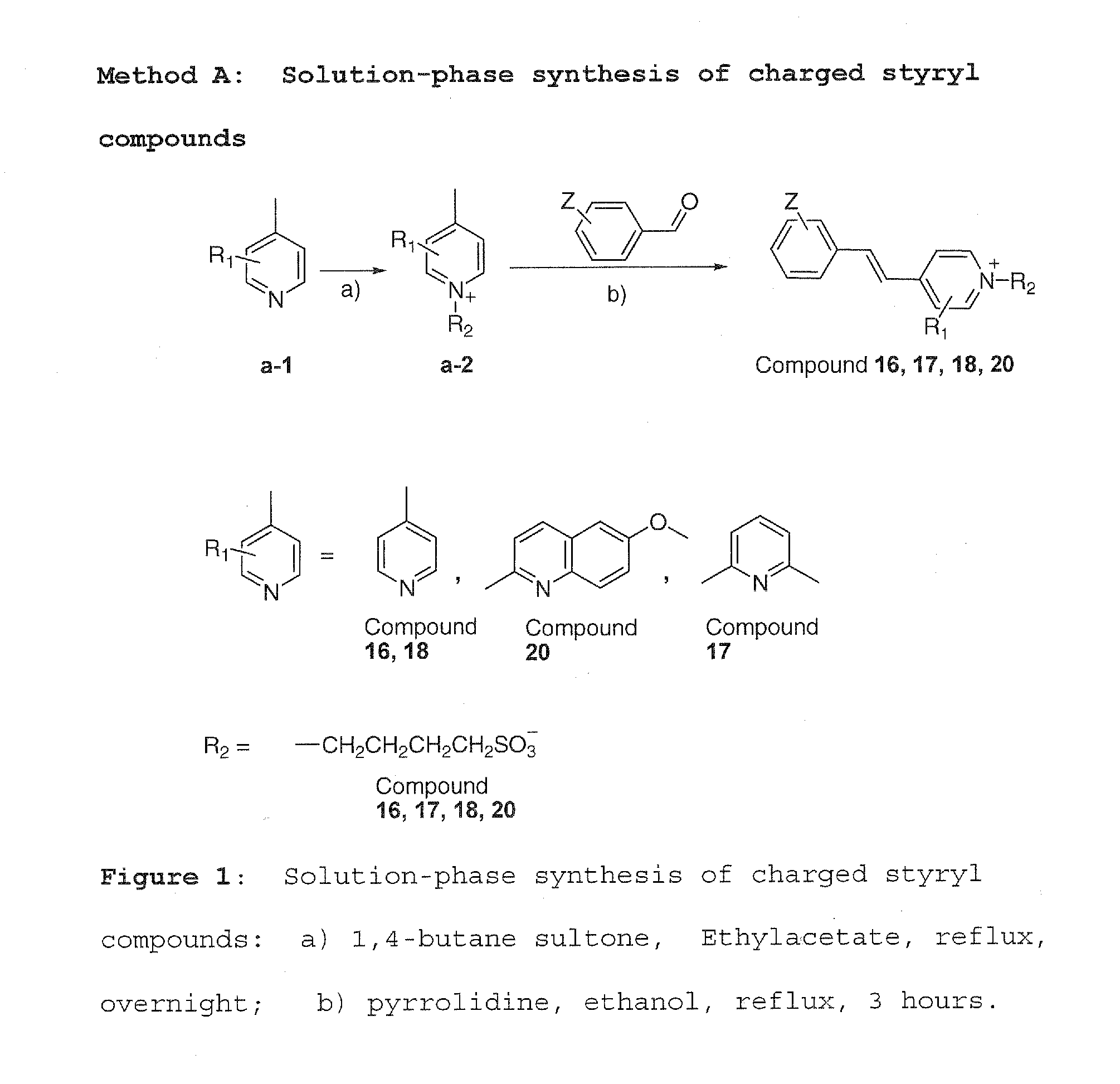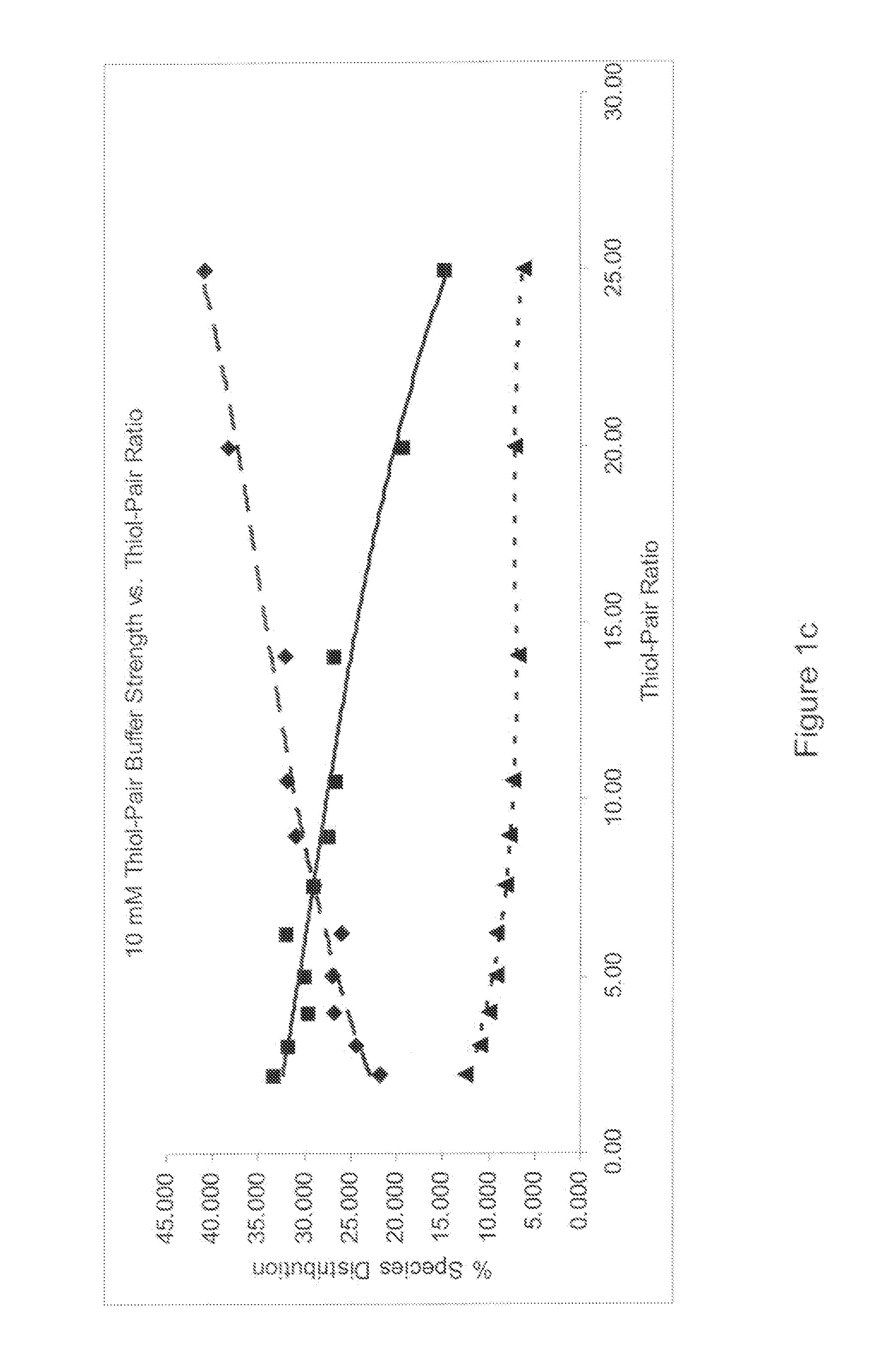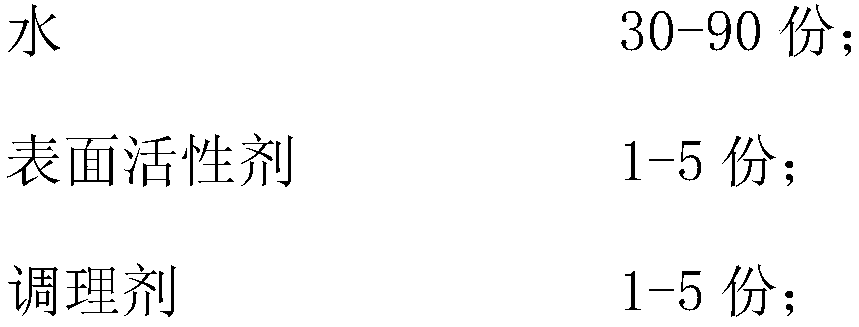Patents
Literature
79 results about "Folding protein" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Refolding of Recombinant Proteins
InactiveUS20080125580A1Peptide/protein ingredientsPeptide preparation methodsHeterologousMolecular biology
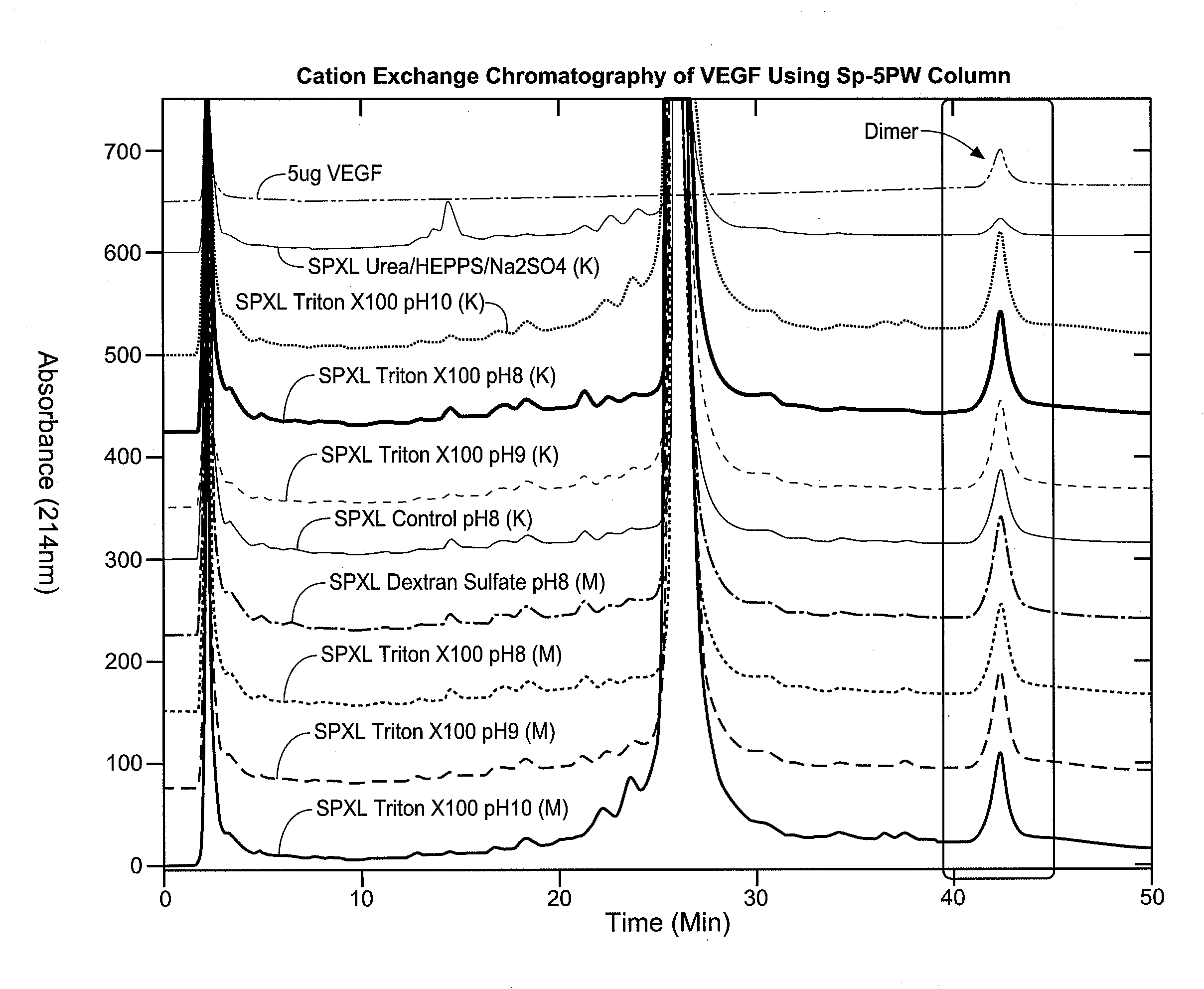
Processes are provided for recovering and purifying refolded recombinant proteins produced in heterologous host cells, which includes the step of refolding the protein in a high pH buffer.
Owner:GENENTECH INC
Methods for drug delivery comprising unfolding and folding proteins and peptide nanoparticles
ActiveUS20110059181A1Reduce the amount requiredImproved cost-efficiencyBiocidePowder deliveryNanoparticleActive agent
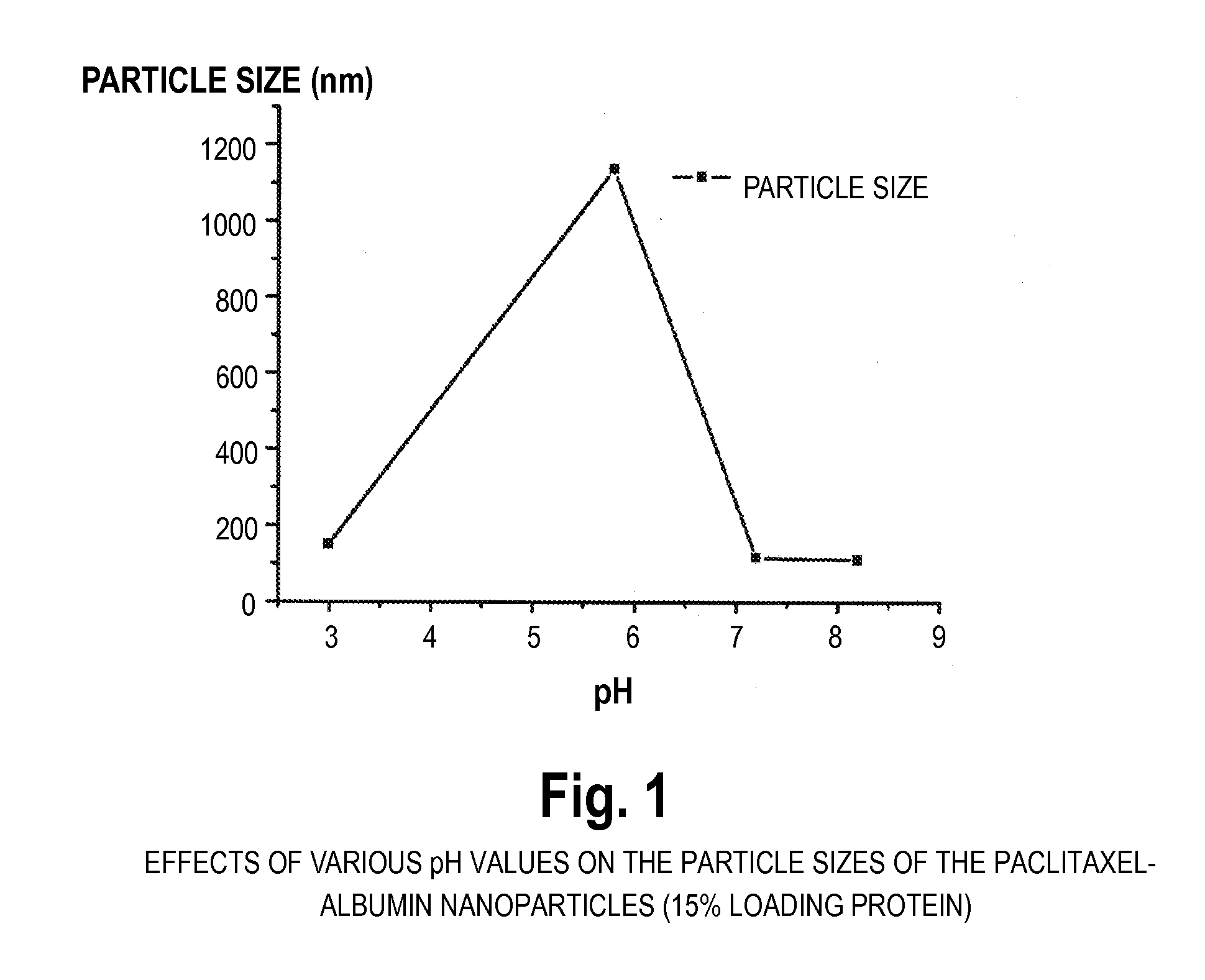
The present invention provides preparation methods of protein nanoparticles for in vivo delivery of pharmacologically active agents, wherein said methods are to encase pharmaceutically active agents into proteins or peptides to form nanoparticles by unfolding the protein, and subsequently refolding or assembling the protein to produce a pharmacologically active agent encased within a protein nanoparticle.
Owner:NANJING UNIV +1
Novel genes, compositions, and methods for modulating the unfolded protein response
InactiveUS20050250182A1Increased transactivation potentialIncrease volumeSugar derivativesHydrolasesAutoimmune conditionAutoimmune disease
The present invention relates to methods and compositions for modulating the unfolded protein response. The method further relates to methods and compositions for the treatment and diagnosis of protein conformational diseases or disorders, including, but not limited to, α1-antitrypsin deficiency, cystic fibrosis, and autoimmune diseases and disorders. The invention further provides methods for modulating the unfolded protein response by modulating XBP1 mRNA splicing.
Owner:UNIV OF MICHIGAN THE
Correctly folded etanercept in high purity and excellent yield
InactiveUS20140072560A1Maximize stabilityMaximize solubilityPeptide/protein ingredientsAntipyreticPresent methodTreatment use
A mixed mode chromatography method for separating correctly folded from incorrectly folded conformations of a given protein is provided. The method is highly effective in separating correctly folded etanercept from incorrectly folded etanercept and aggregates in commercially attractive yields capable of affording etanercept preparations having very high purity in terms of correctly folded etanercept versus incorrectly folded etanercept. The invention is further directed to protein preparations and formulations comprising correctly folded proteins obtained using the present methods, and methods of treatment using the high purity preparations obtained from the mixed mode method.
Owner:COHERUS BIOSCI
Conductance of Improperly Folded Proteins Through the Secretory Pathway And Related Methods For Treating Disease
InactiveUS20080025921A1Improve breathabilityReduce concentrationHalogenated hydrocarbon active ingredientsBiocideReticulum cellMedicine
This invention provides the methodology and agents for treating any disease or clinical condition which is at least partly the result of endoplasmic reticulum-associated retention of proteins. Thus, the methods and agents of the present invention provide for the release of normally retained proteins from the endoplasmic reticulum. The present invention is particularly useful for treating any disease or clinical condition which is at least partly the result of endoplasmic reticulum-associated retention or degradation of mis-assembled or mis-folded proteins.
Owner:N FOLD LLC
Engineering of leader peptides for the secretion of recombinant proteins in bacteria
InactiveUS7419783B2Increased green fluorescent protein fluorescenceImproved protein exportBacteriaPeptide/protein ingredientsBacteroidesADAMTS Proteins
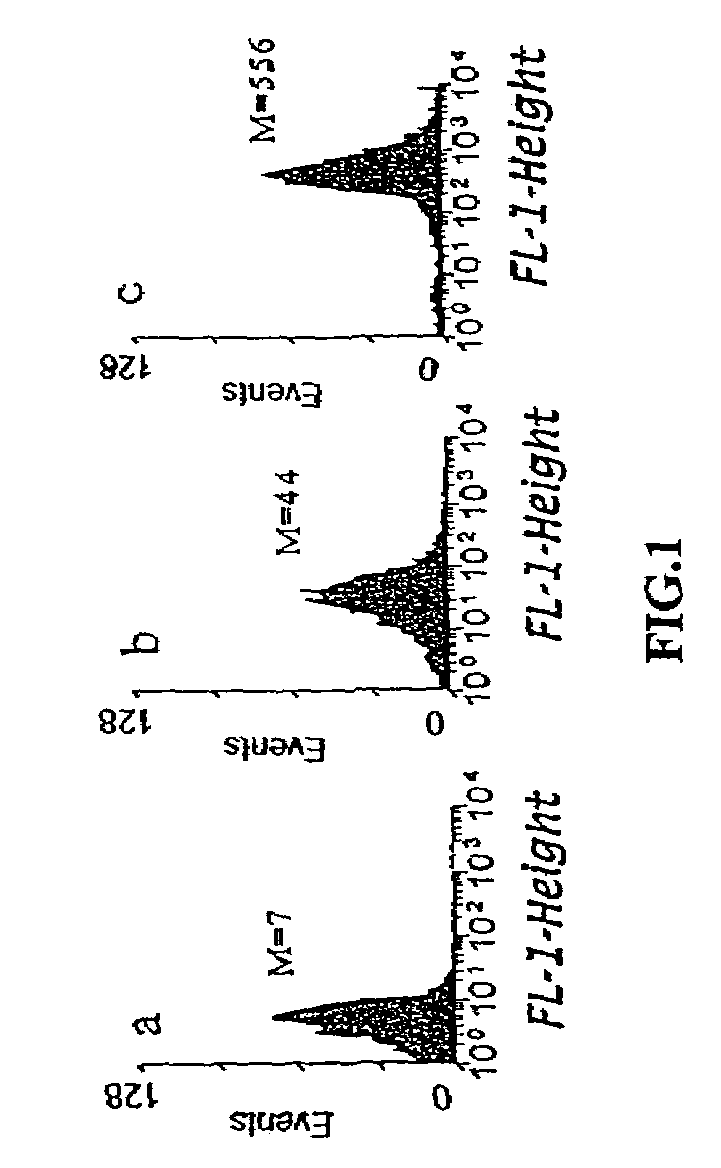
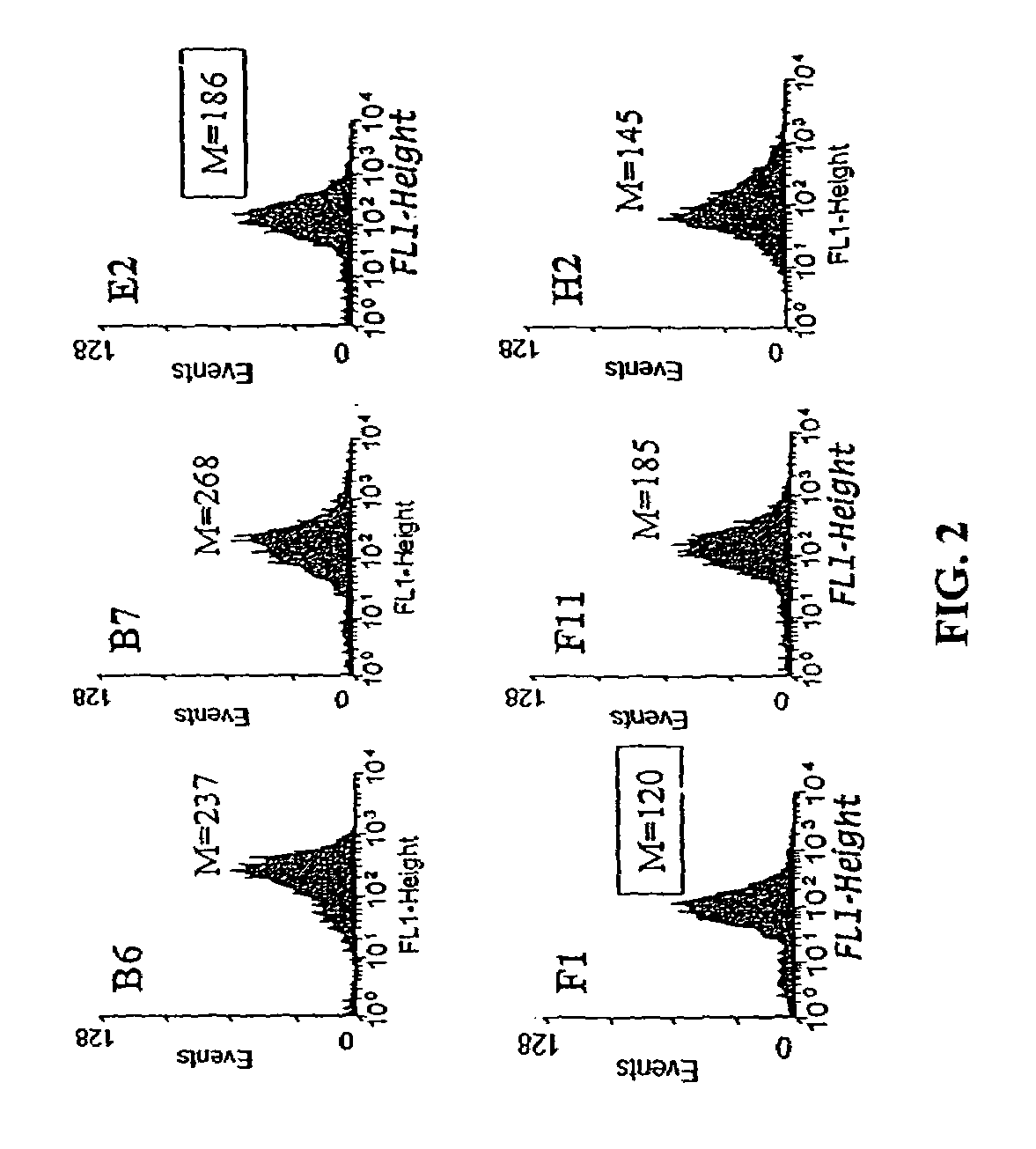
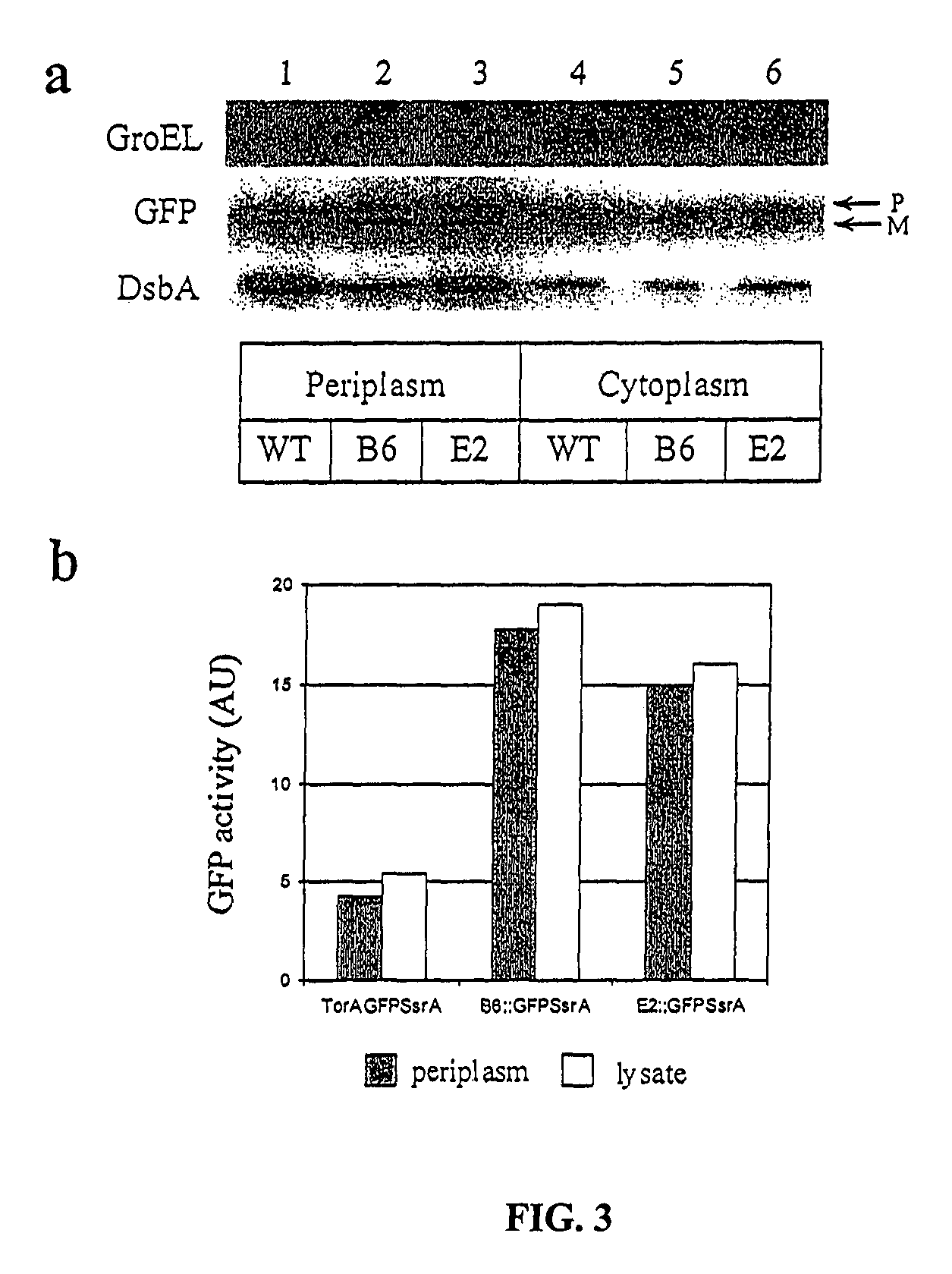
The present invention provides methods of isolating of leader peptides capable of directing export of heterologous proteins from the bacterial cytoplasm. The methods rely on the screening of libraries of putative leader peptides or of leader peptide mutants for sequences that allow rapid export and thus can rescue a short-lived reporter protein from degradation in the cytoplasm. The mutant leader peptides identified herein are shown to confer significantly higher steady state levels of export not only for short-lived reporter protein but also for other stable, long-lived proteins. These leader peptides can be used to direct or enhance protein secretion. The present invention further discloses methods for the export of cytoplasmically folded protein via the Tat pathway. Proteins having disulfide bonds are first folded within the cytoplasm in suitable oxidizing mutant strains. Such cytoplasmically pre-folded proteins containing disulfide bonds are then exported via the Tat pathway.
Owner:RES DEVMENT FOUND
Imaging agents for protein misfolding
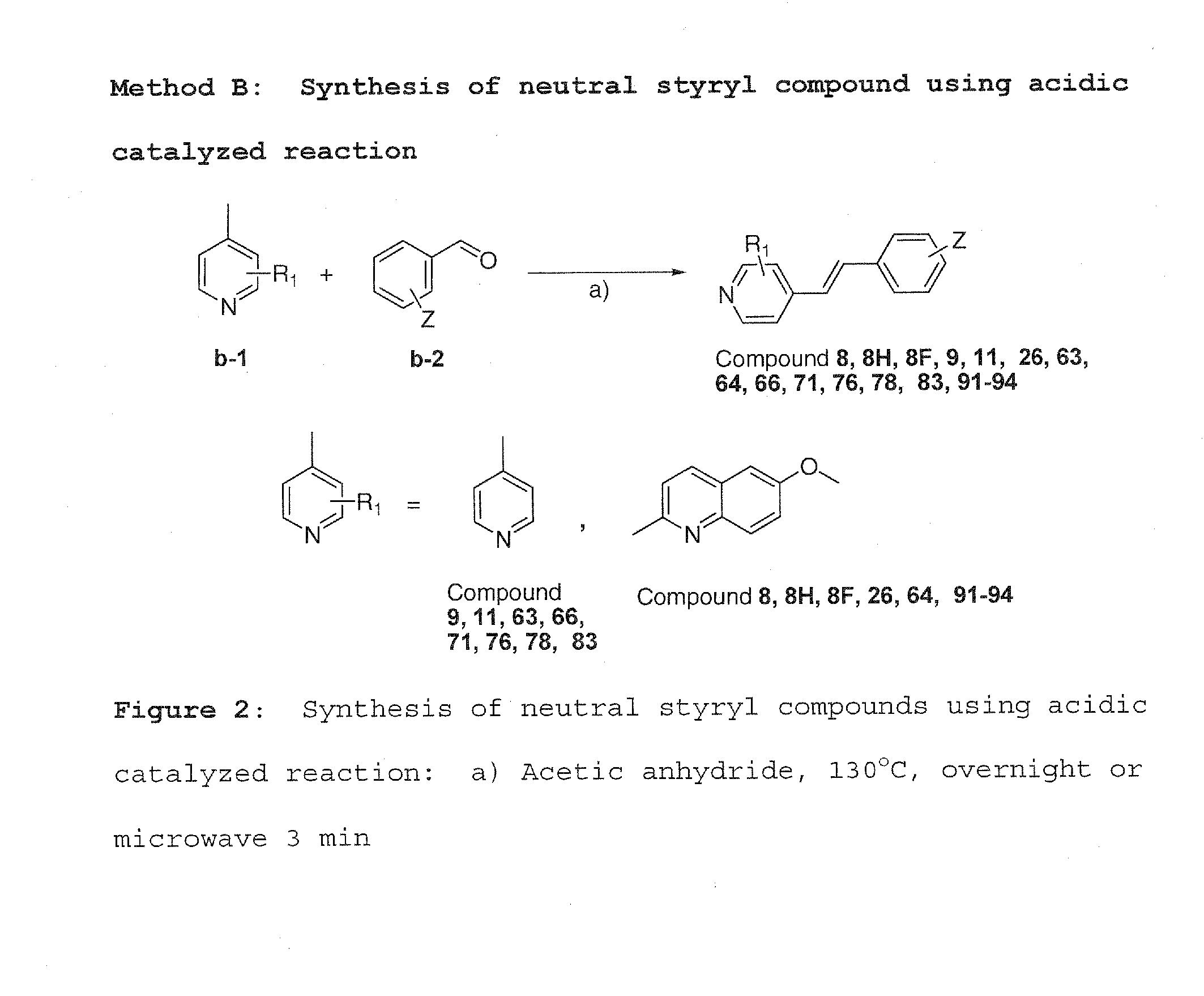
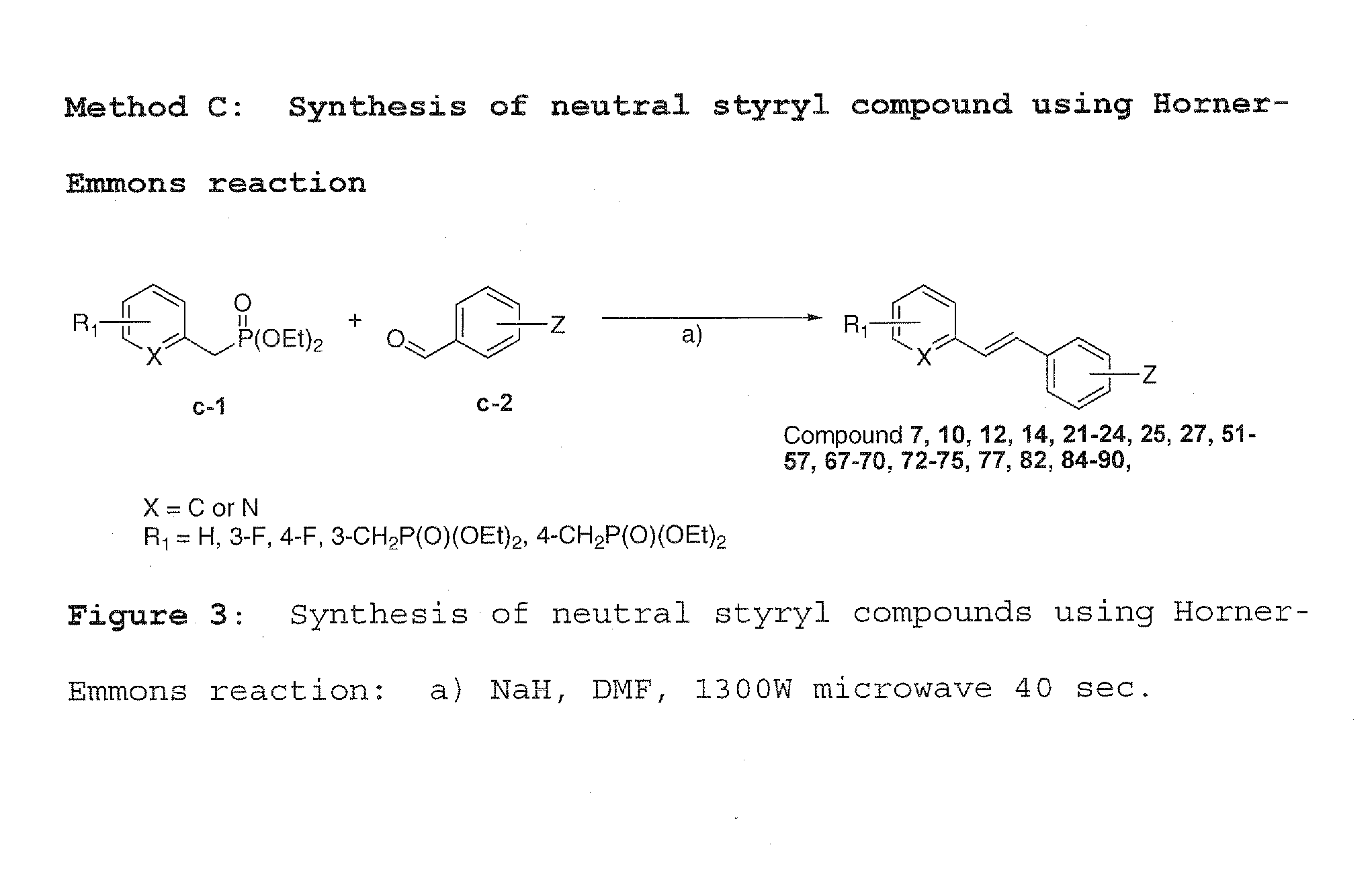
Charged and neutral small fluorescent molecules based upon the styryl scaffold are useful as imaging agents for misfolded proteins such as amyloid plaque. Charged molecules are prepared using pyrrolidine catalyzed reactions by solution-phase synthesis. Neutral styryl molecules are prepared using acetic anhydride catalyzed reactions, Horner-Emmons reactions or Wittig reactions.
Owner:NEW YORK UNIV
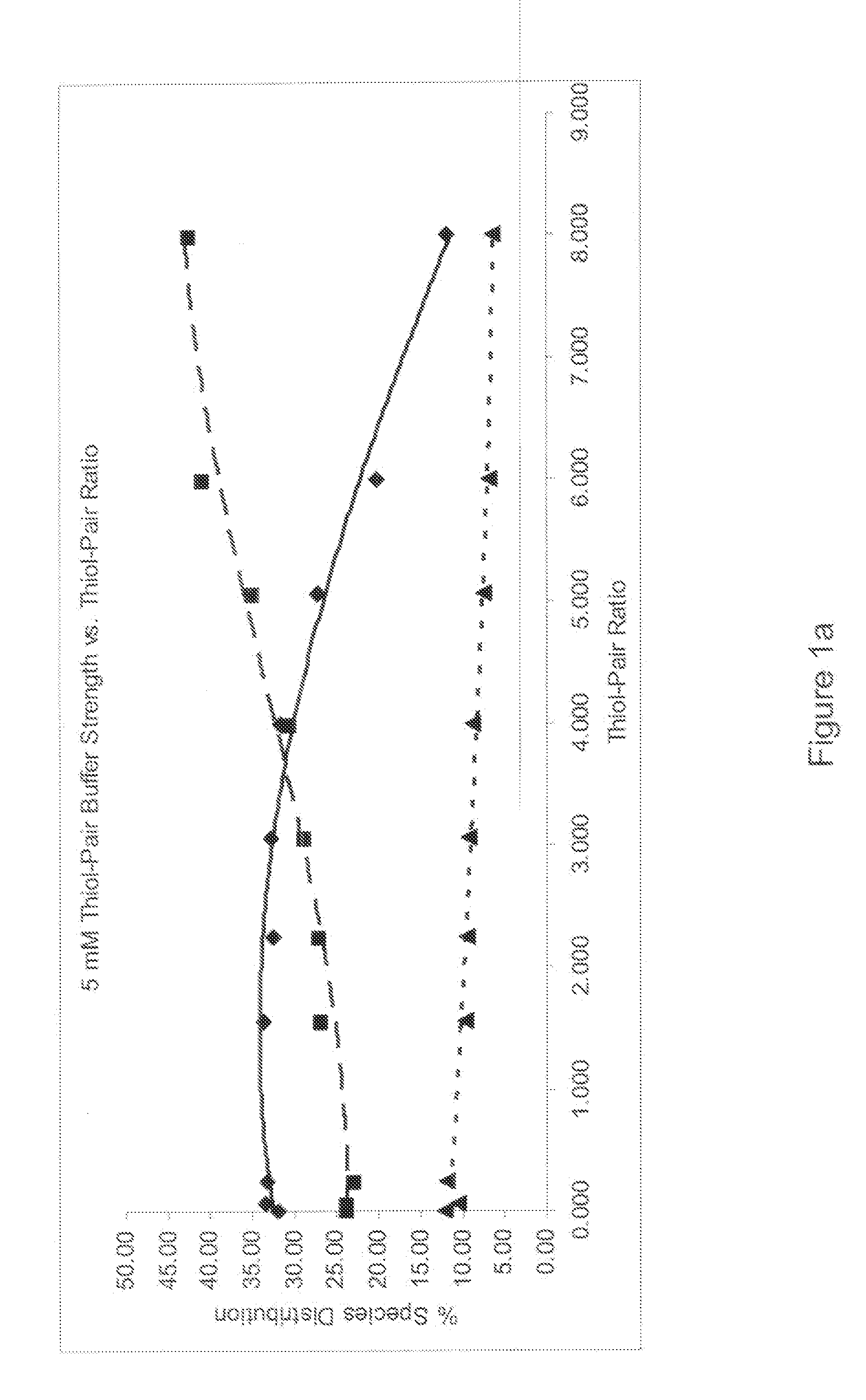
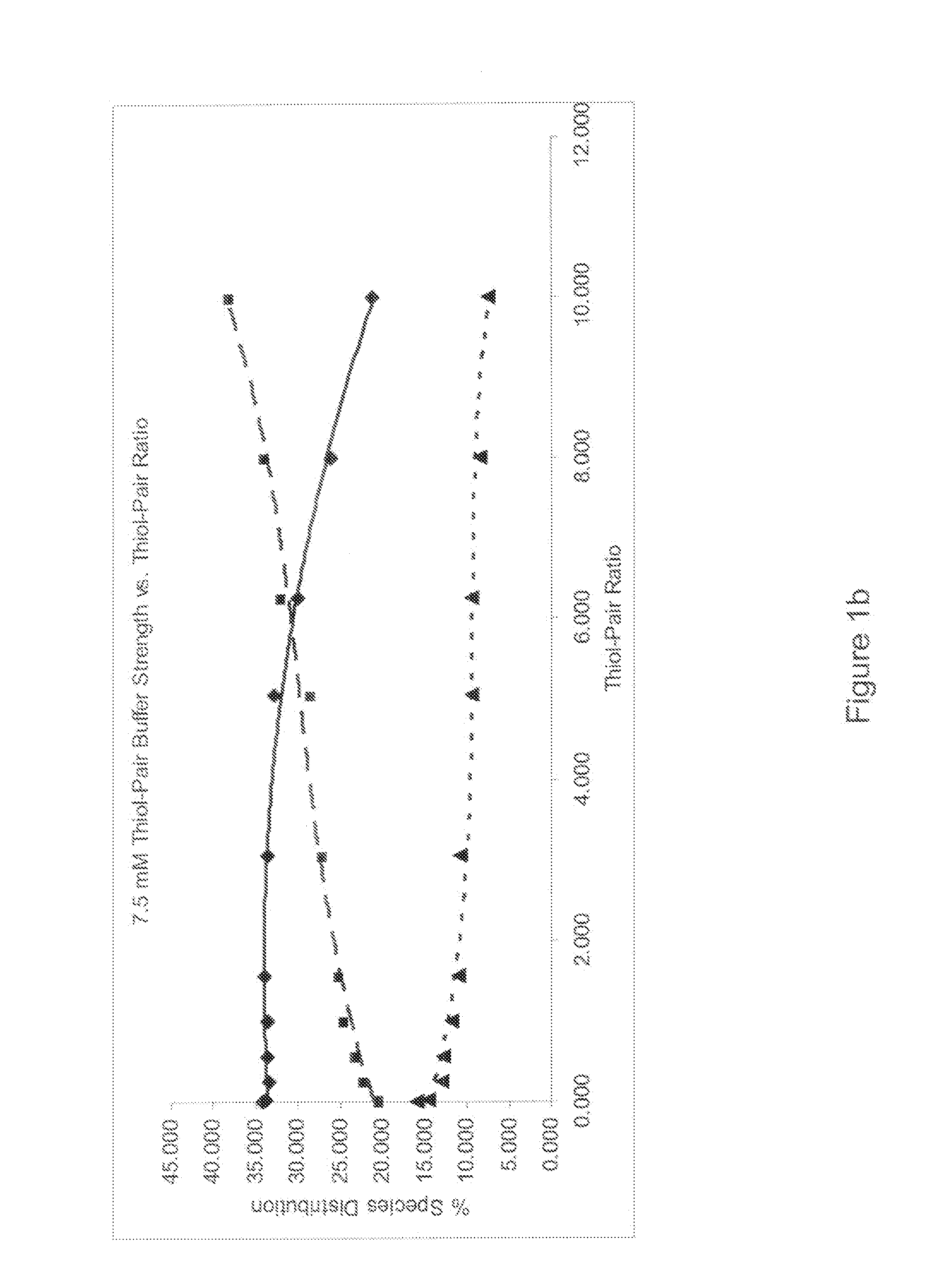
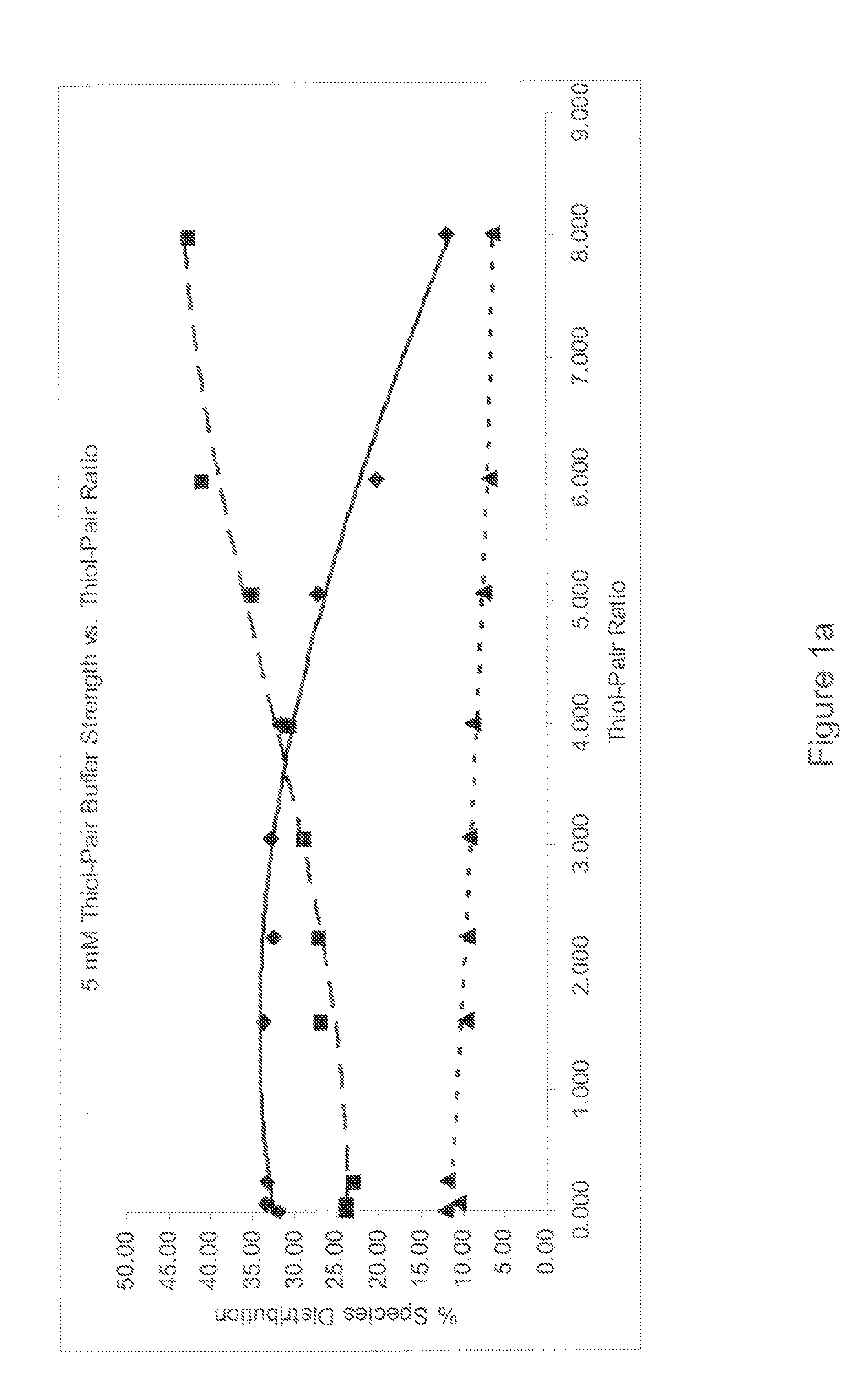
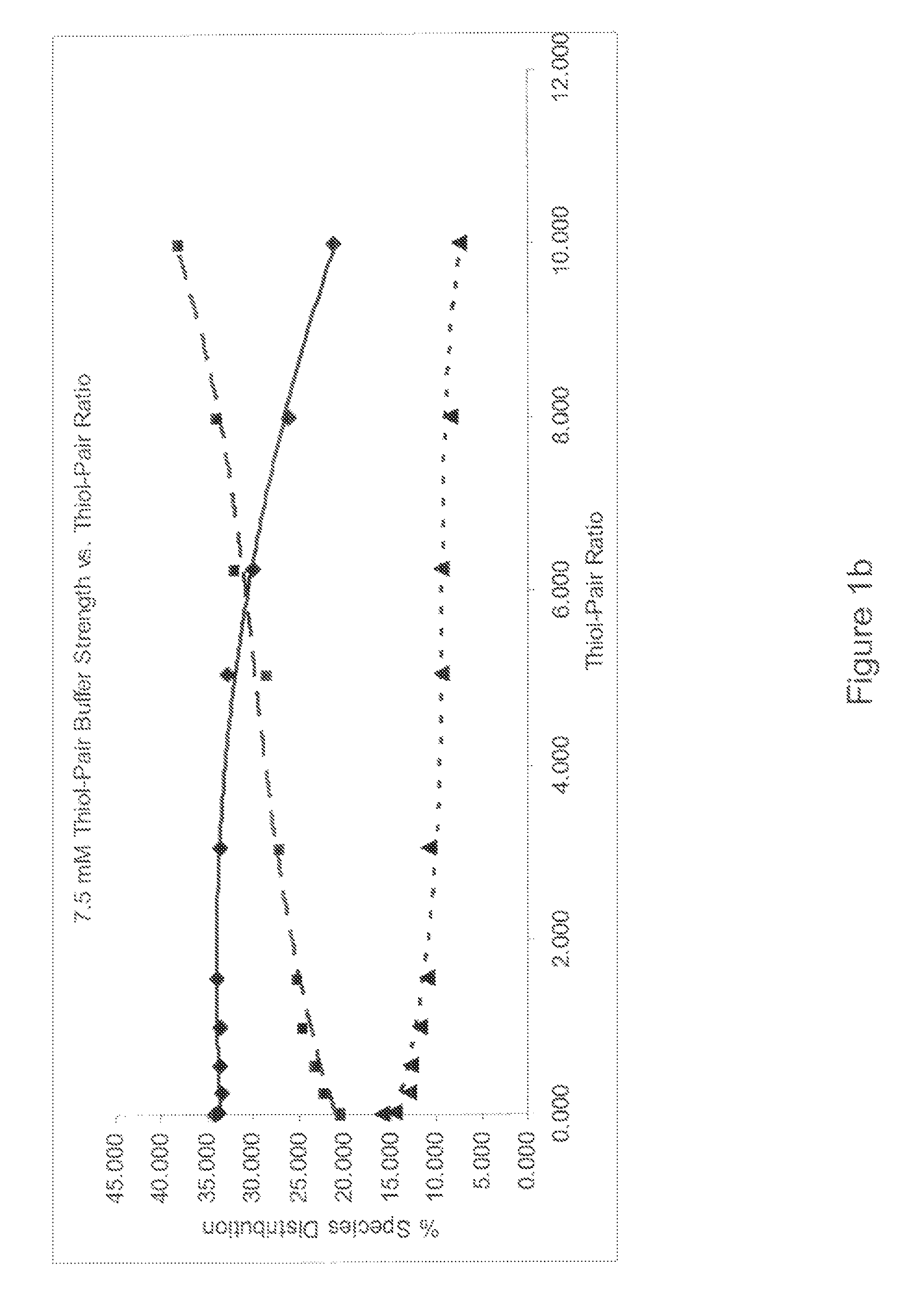
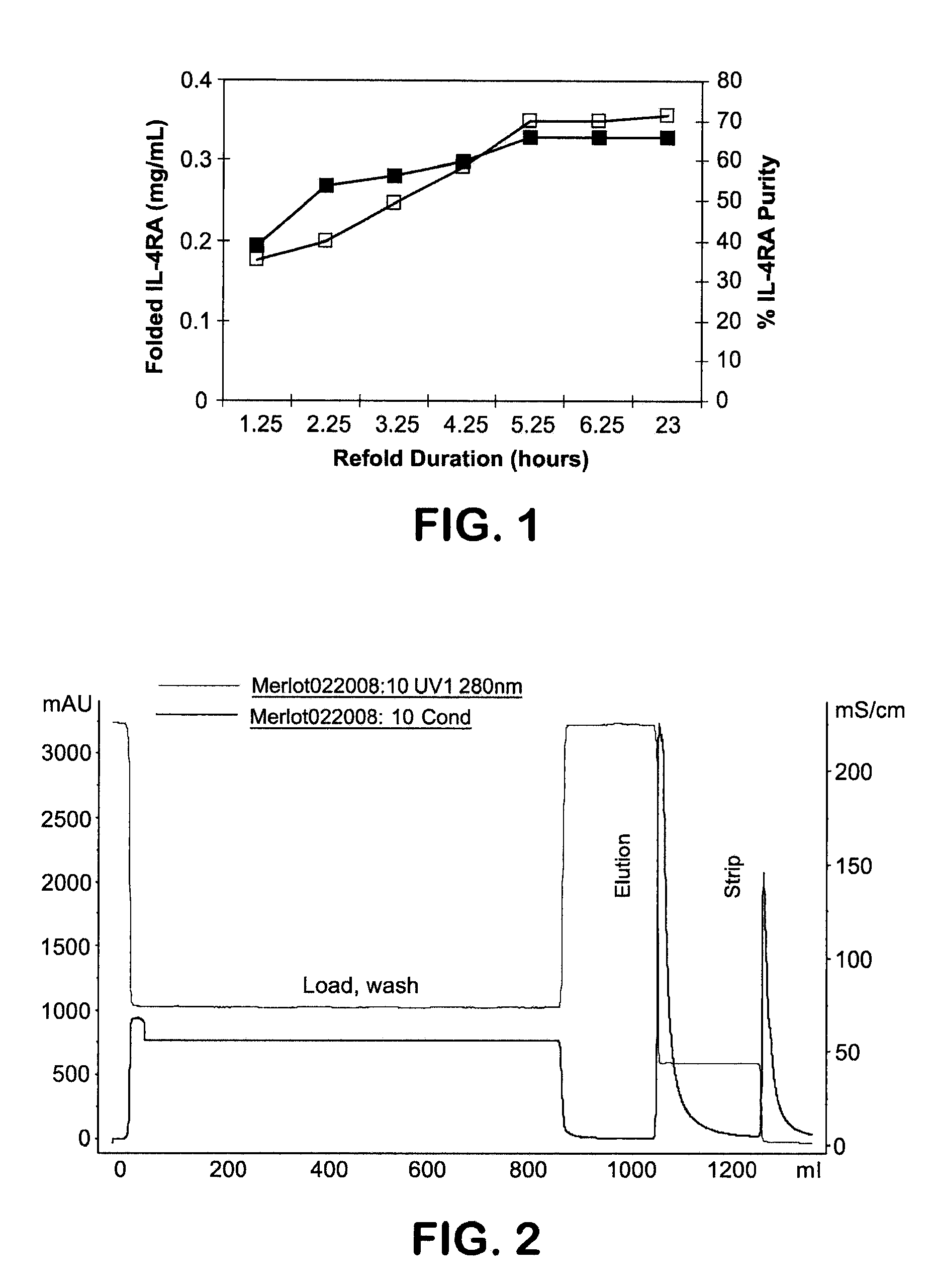
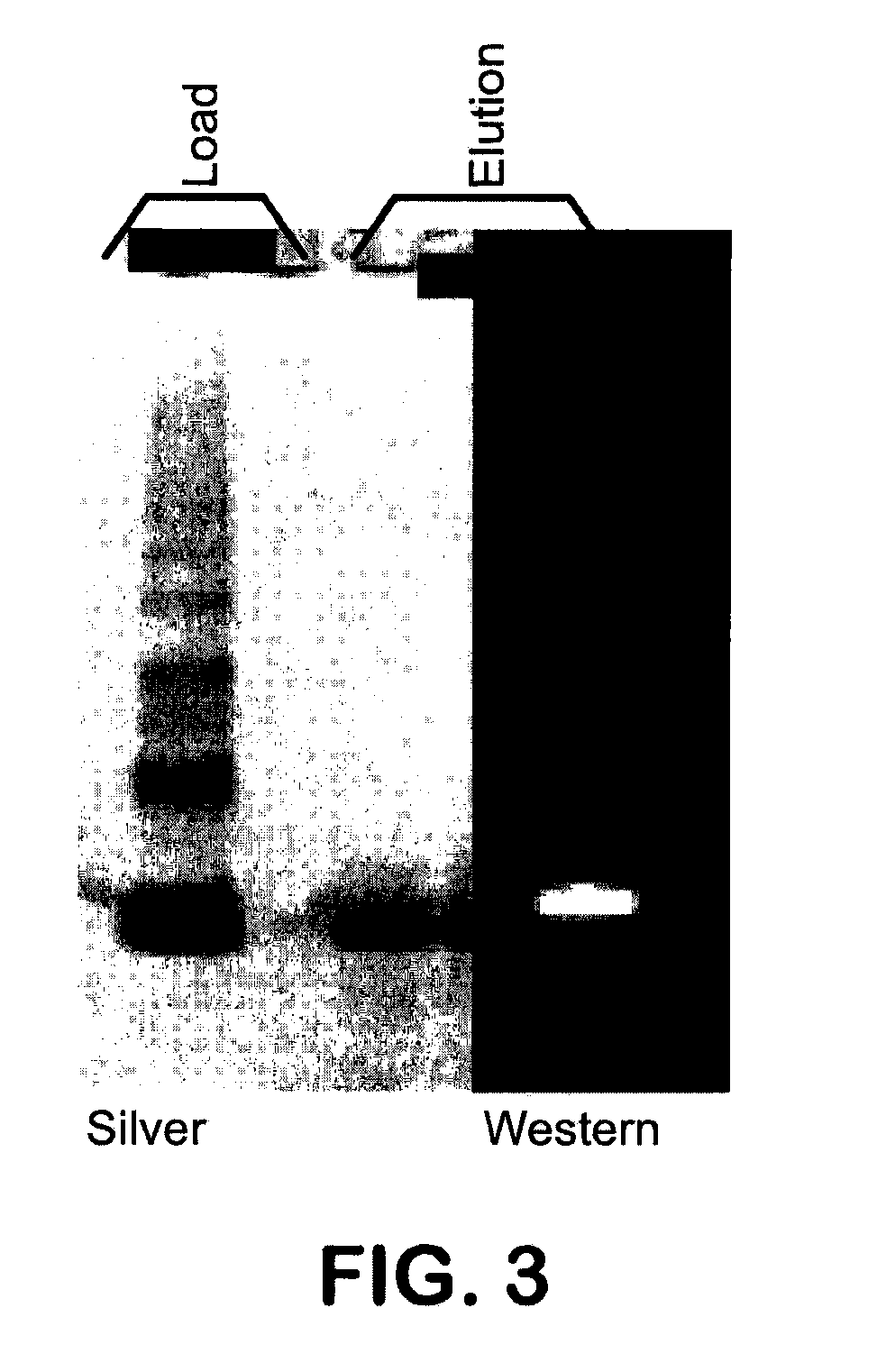
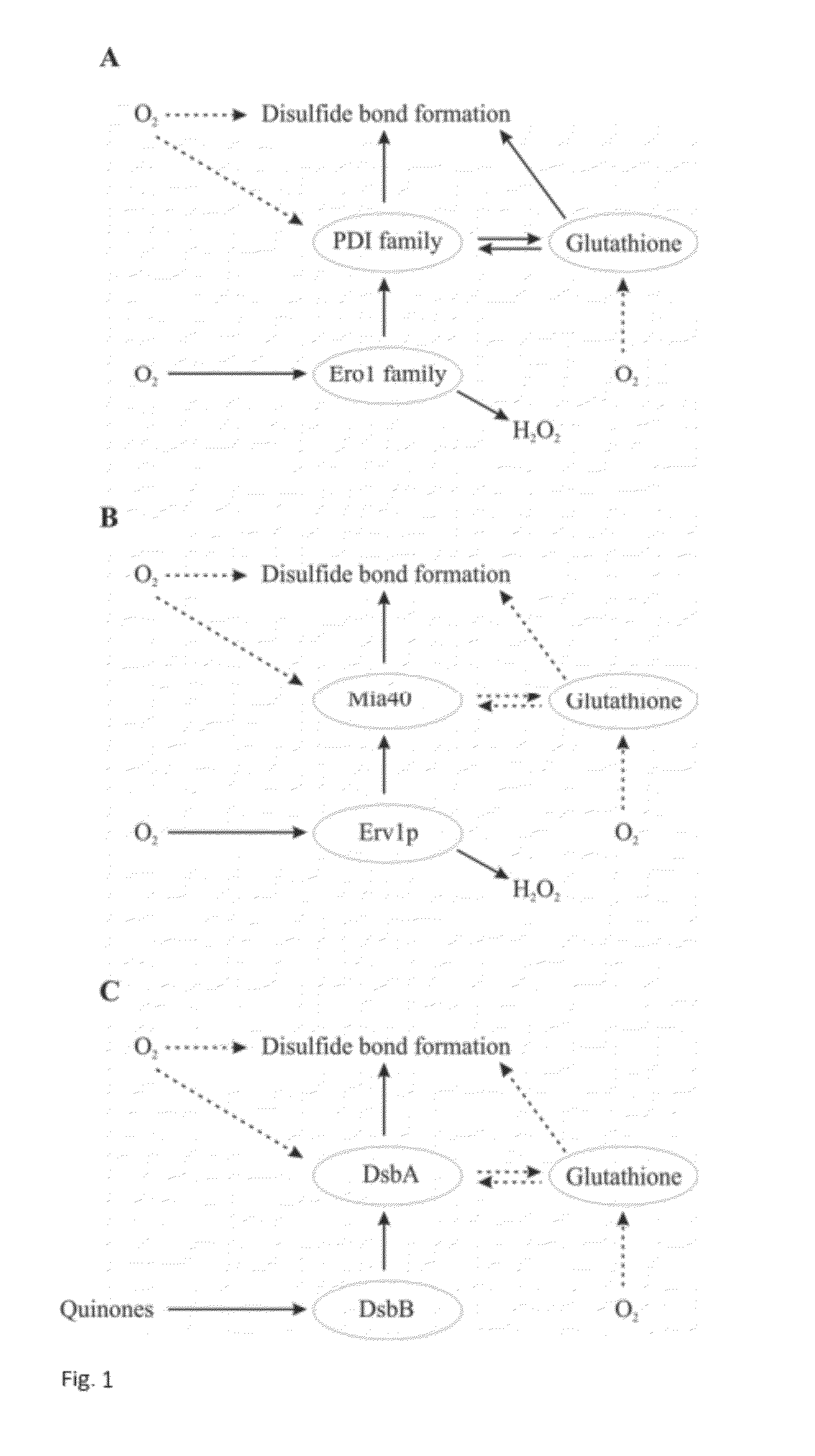
Refolding proteins using a chemically controlled redox state
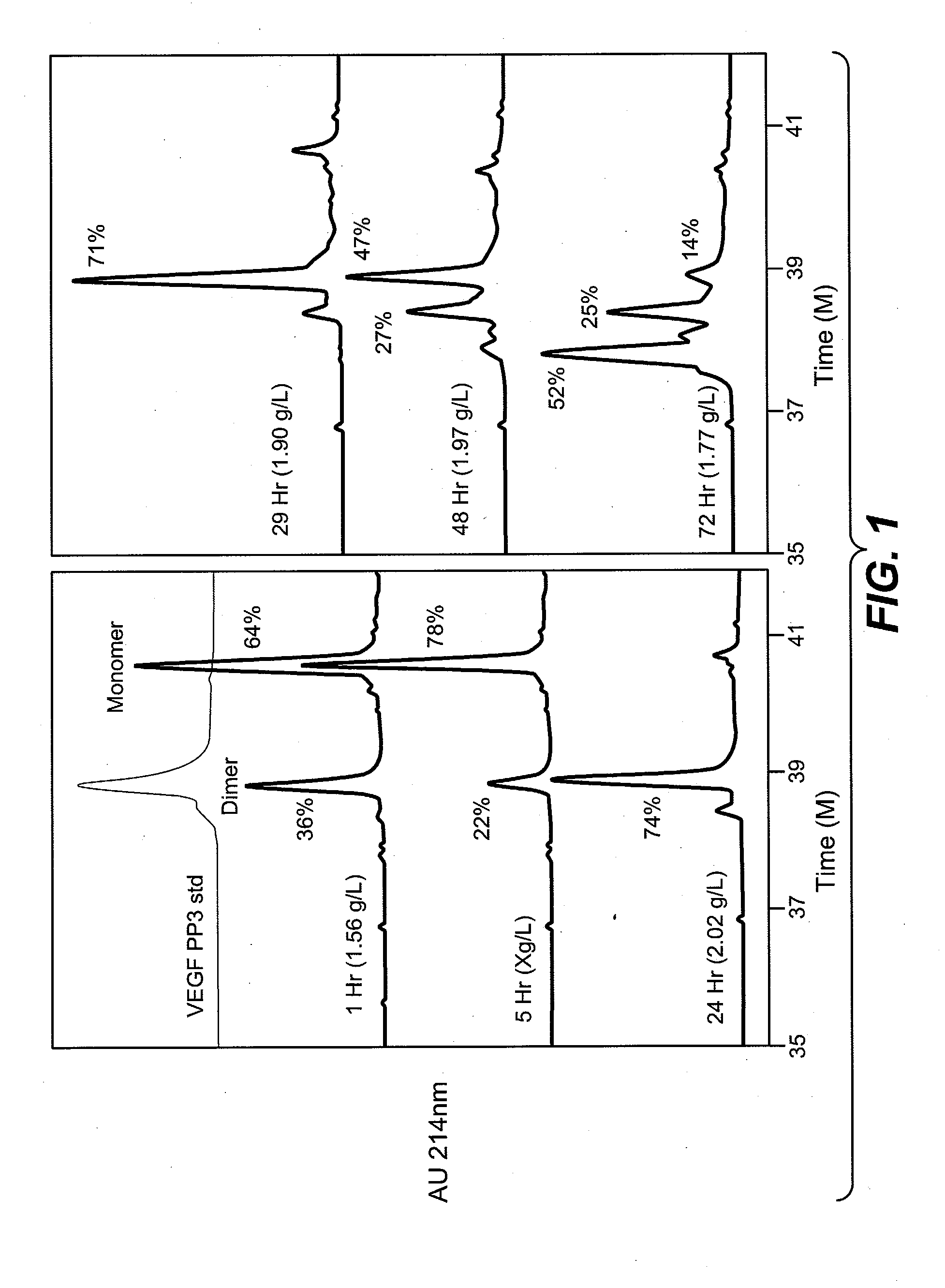
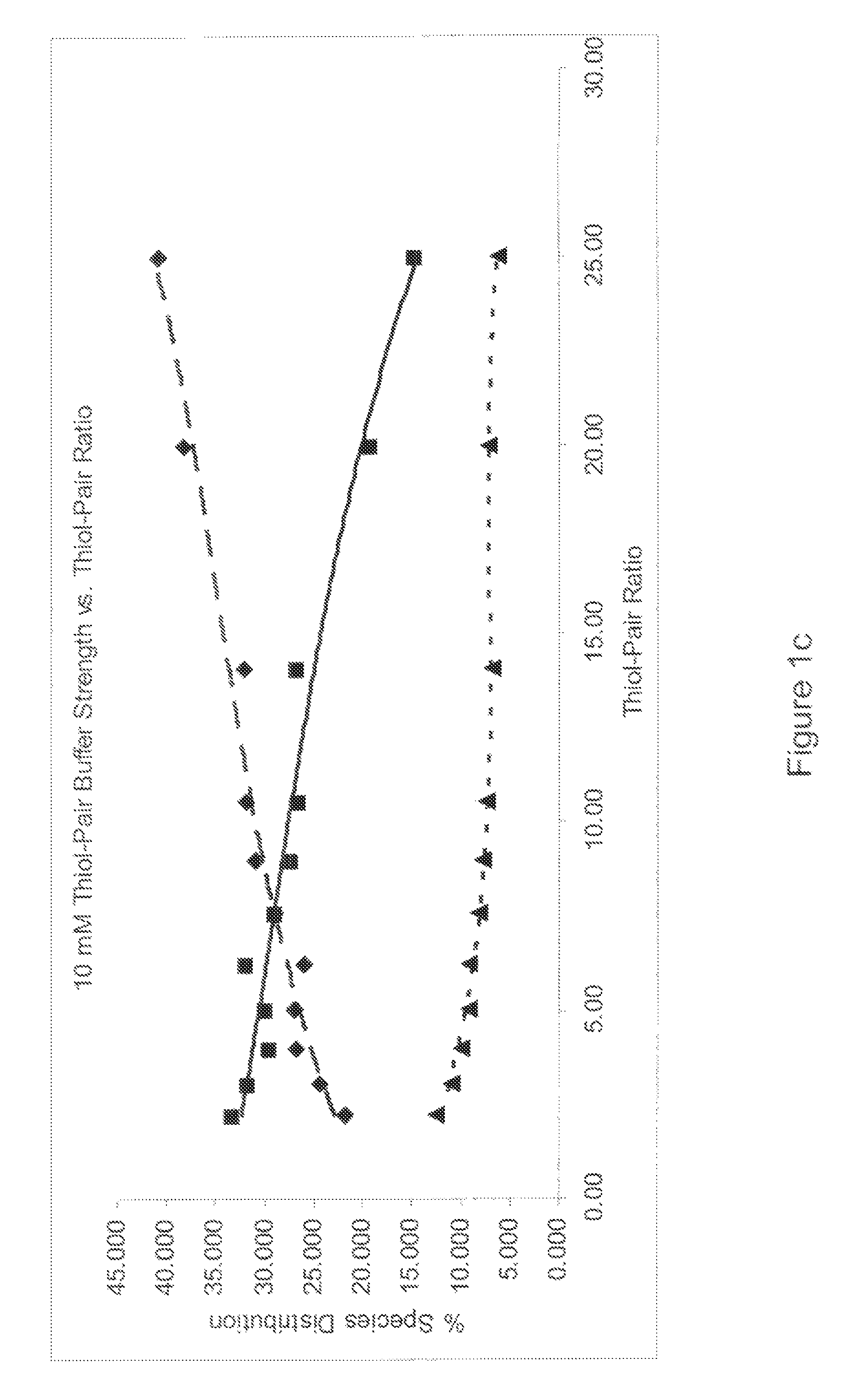
A method of refolding proteins expressed in non-mammalian cells present in concentrations of 2.0 g / L or higher is disclosed. The method comprises identifying the thiol pair ratio and the redox buffer strength to achieve conditions under which efficient folding at concentrations of 2.0 g / L or higher is achieved and can be employed over a range of volumes, including commercial scale.
Owner:AMGEN INC
Protein coupling method based on catenane
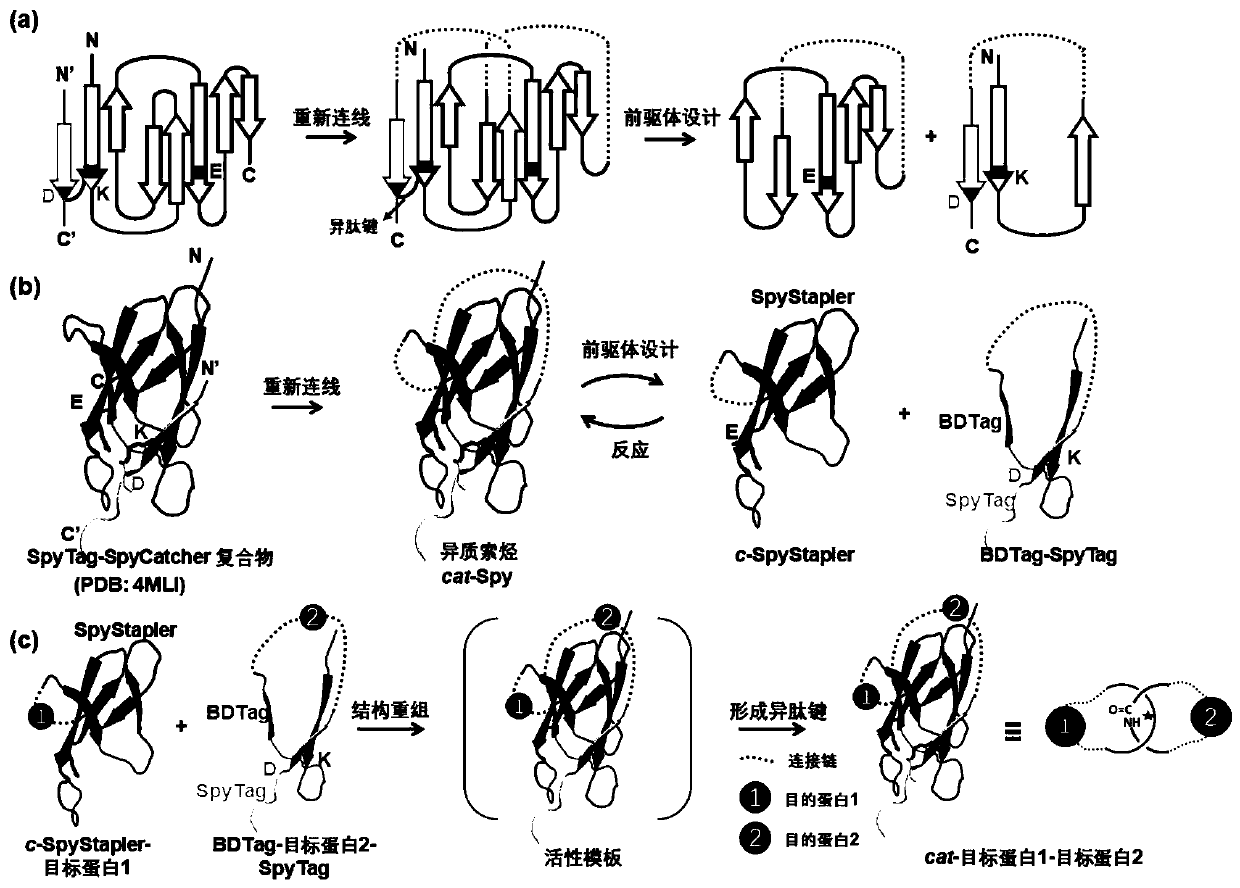
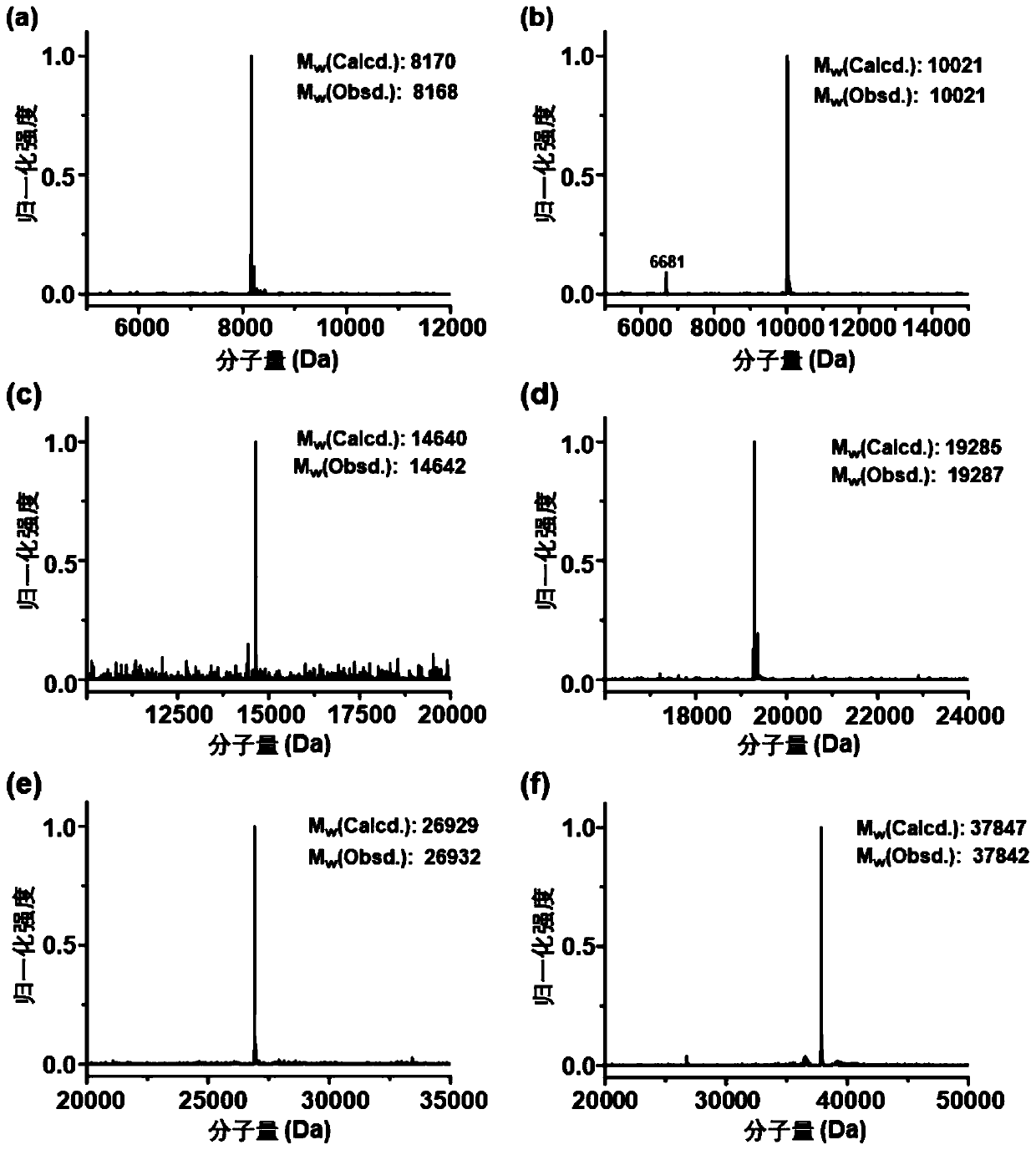
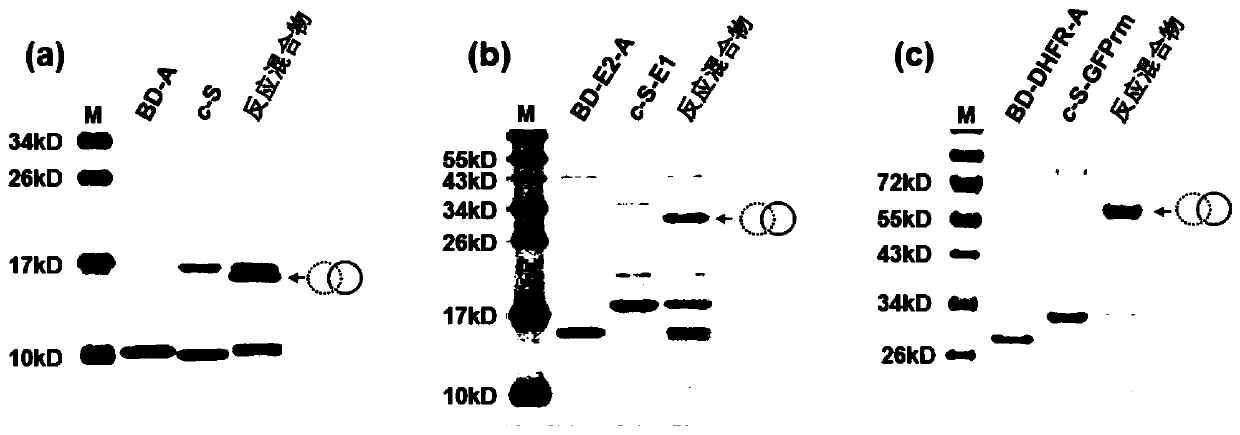
ActiveCN110272913AAchieving concise compositionImprove resistance to enzymatic hydrolysisBacteriaMicroorganism based processesProtein targetCoupling
The invention discloses a protein coupling method based on catenane. Each second-level component (SpyTag, BDTag and SpyStapler) in a SpyTag-SpyCatcher compound is connected renewedly, a chain entanglement structure designed by labor is introduced, a protein activity template method combining winding and catalyzing is developed, and mechanical key coupling of different target proteins connected in a catenane mode can be realized. The protein coupling method based on the catenane is used for performing heterogeneous catenane on disordered proteins or folding proteins, and the structure and performance of the proteins cannot be affected. The catenane process further can realize intracellular direct expression preparation through a coexpression system. Due to the fact that a catenane structure has a conformation limiting effect and space control on the target proteins, and the stability of the target proteins can be improved. According to the protein coupling method based on the catenane, concise synthesis of protein heterogeneous catenane is realized, and the protein coupling method based on the catenane is a very valuable and novel protein coupling strategy.
Owner:PEKING UNIV
Genes encoding for genetic stability, gene expression and folding proteins
InactiveUS7138513B2High expressionIncrease productionSugar derivativesBacteriaMicroorganismGene expression
The invention relates to novel nucleic acid molecules, to the use thereof for constructing genetically improved microorganisms and to methods for preparing fine chemicals, in particular amino acids, with the aid of said genetically improved microorganisms.
Owner:DAESANG CORP
Methods for structural analysis of proteins
InactiveUS7288382B2Overcome limitationsEffective regulationDepsipeptidesPeptide preparation methodsStructure analysisBinding site
The invention provides methods and compositions for protein structure analysis, including substrate binding sites, sites of protein-protein interactions, three dimensional structure analysis, and stability, all with single amino acid resolution. In general, the subject methods involve introduction of cysteine residues, which serve as probes for physical analysis, into a protein by translational misincorporation in vivo. In many embodiments, proteins containing misincorporated cysteine residues are reacted with a crosslinking agent that covalently links misincorporated cysteine residues to a proximal amino acid in the folded protein. These methods, termed “MXLINK” methods, may be used for protein tertiary structure analysis. In other embodiments, cysteine-misincorporated proteins are used in protein footprinting methods, termed “MPAX” or “MSX” methods.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
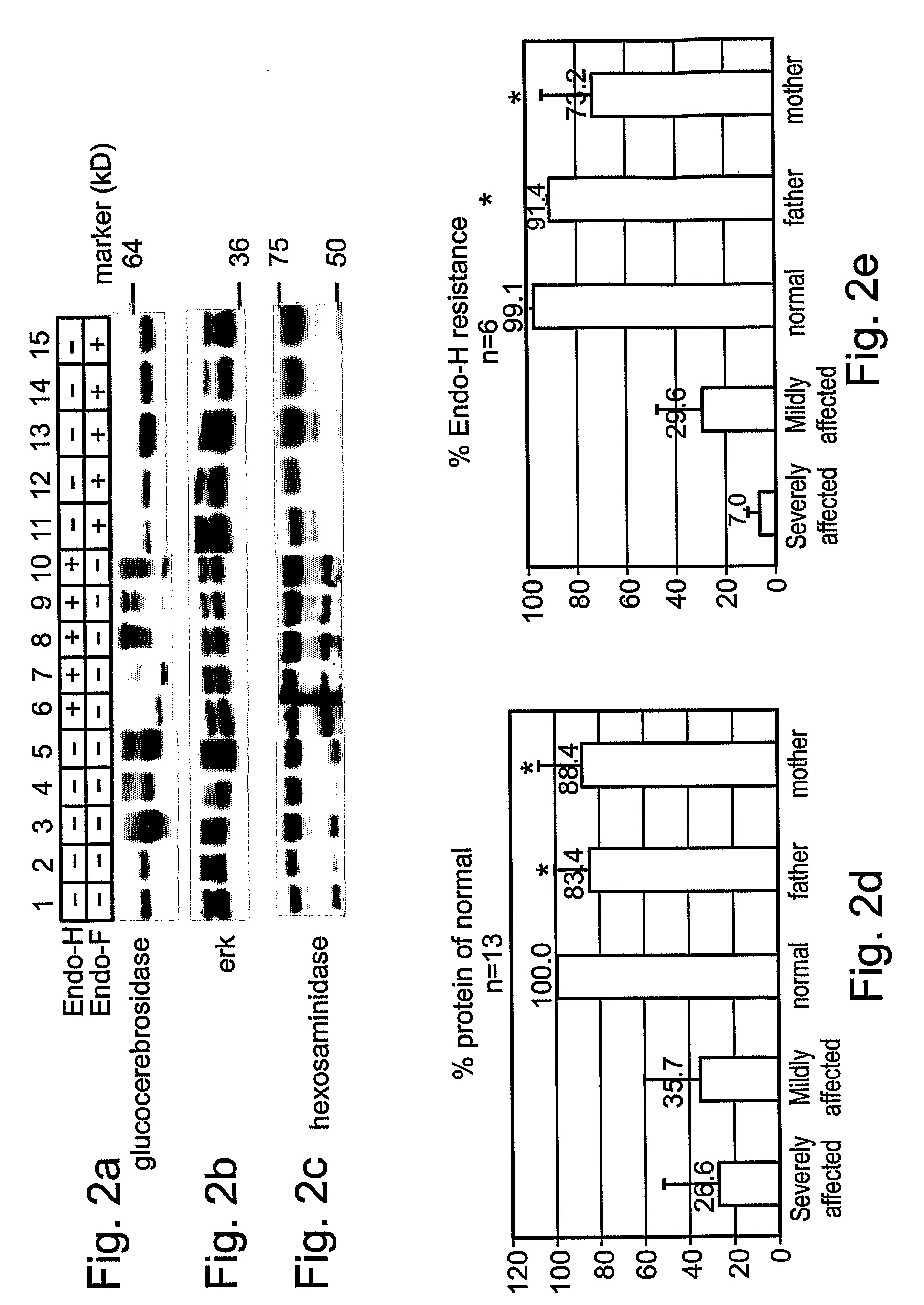
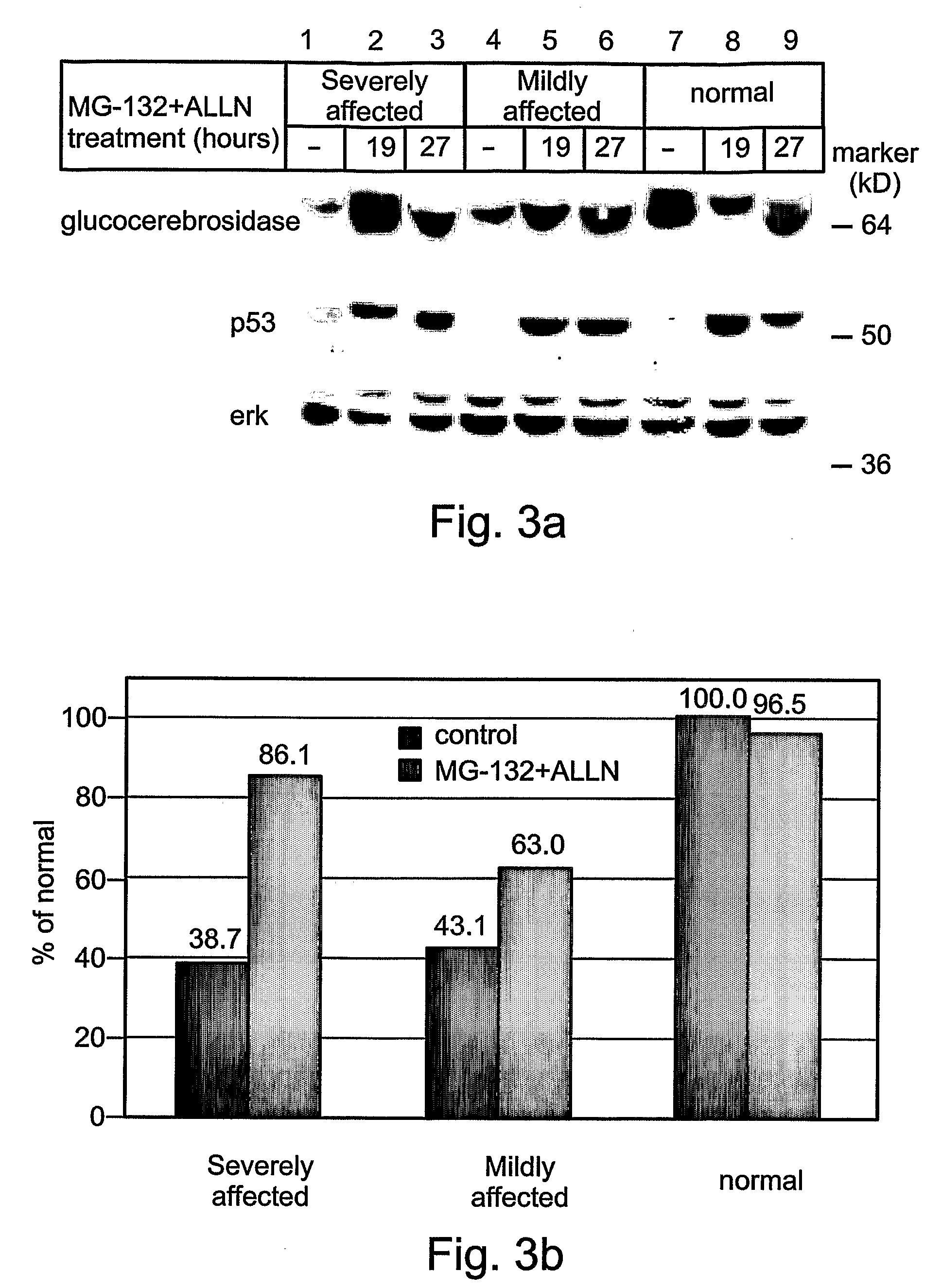
Methods and kits for diagnosing and/or assessing severity and treating gaucher disease
InactiveUS20090239807A1Improve the level ofMicrobiological testing/measurementTetrapeptide ingredientsLysosomeTreatment hypertension
Methods and kits for treating Gaucher disease are provided. The methods are based on using agents capable of inhibiting proteasomal degradation of glucocerebrosidase and / or elevating a level of mis-folded yet active glucocerebrosidase in cell lysosomes. Also provided are methods and kits for diagnosing and / or assessing a severity and determining prognosis of Gaucher disease or other diseases associated with abnormally folded proteins which are retained in the ER.
Owner:RAMOT AT TEL AVIV UNIV LTD
Refolding proteins using a chemically controlled redox state
A method of refolding proteins expressed in non-mammalian cells present in concentrations of 2.0 g / L or higher is disclosed. The method comprises identifying the thiol pair ratio and the redox buffer strength to achieve conditions under which efficient folding at concentrations of 2.0 g / L or higher is achieved and can be employed over a range of volumes, including commercial scale.
Owner:AMGEN INC
Natural-plant-pigment hair dye and preparation method thereof
InactiveCN108403580AGood skin affinityEnhanced hydrophobic interactionsCosmetic preparationsHair cosmeticsHair dyesPlant Sources
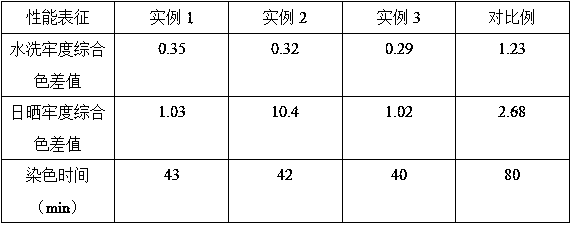
The invention relates to natural-plant-pigment hair dye and a preparation method thereof and belongs to fine chemical engineering. The natural-plant-pigment hair dye has the advantages that sulfydrylis connected to high-safety natural-plant-pigment dye to form active dye; during hair dyeing, disulfide bonds in hair are opened, and the sulfydryl in the active dye and sulfydryl in the hair disulfide bonds connecting together so as to achieve permanent dyeing; meanwhile, the synergic effect of rabbit hair keratin and soybean protein is utilized to fold protein molecules, hydrophobic groups and sulfydryl embedded inside the protein are exposed to enhance biological adhesion, the hydrophobic film of hair epicuticle is promoted to absorb nutritional substances, and a thin film is formed on thesurface of the hair to protect the hair; the natural-plant-pigment hair dye is nontoxic plant-source hair dye free of carcinogenicit and is good in hair dyeing effect and good in washing fastness andlight fastness.
Owner:王敏
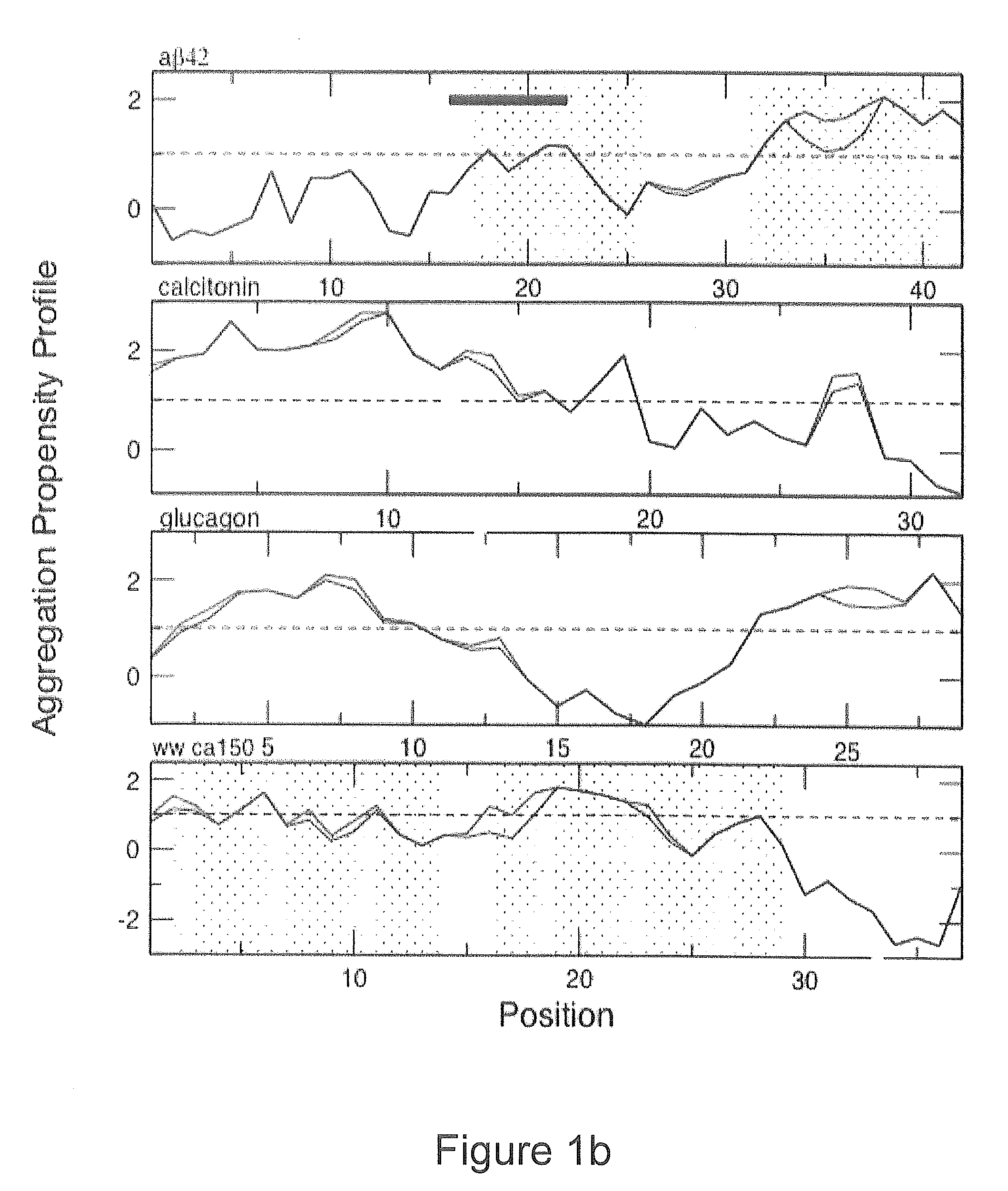
Protein aggregation prediction systems
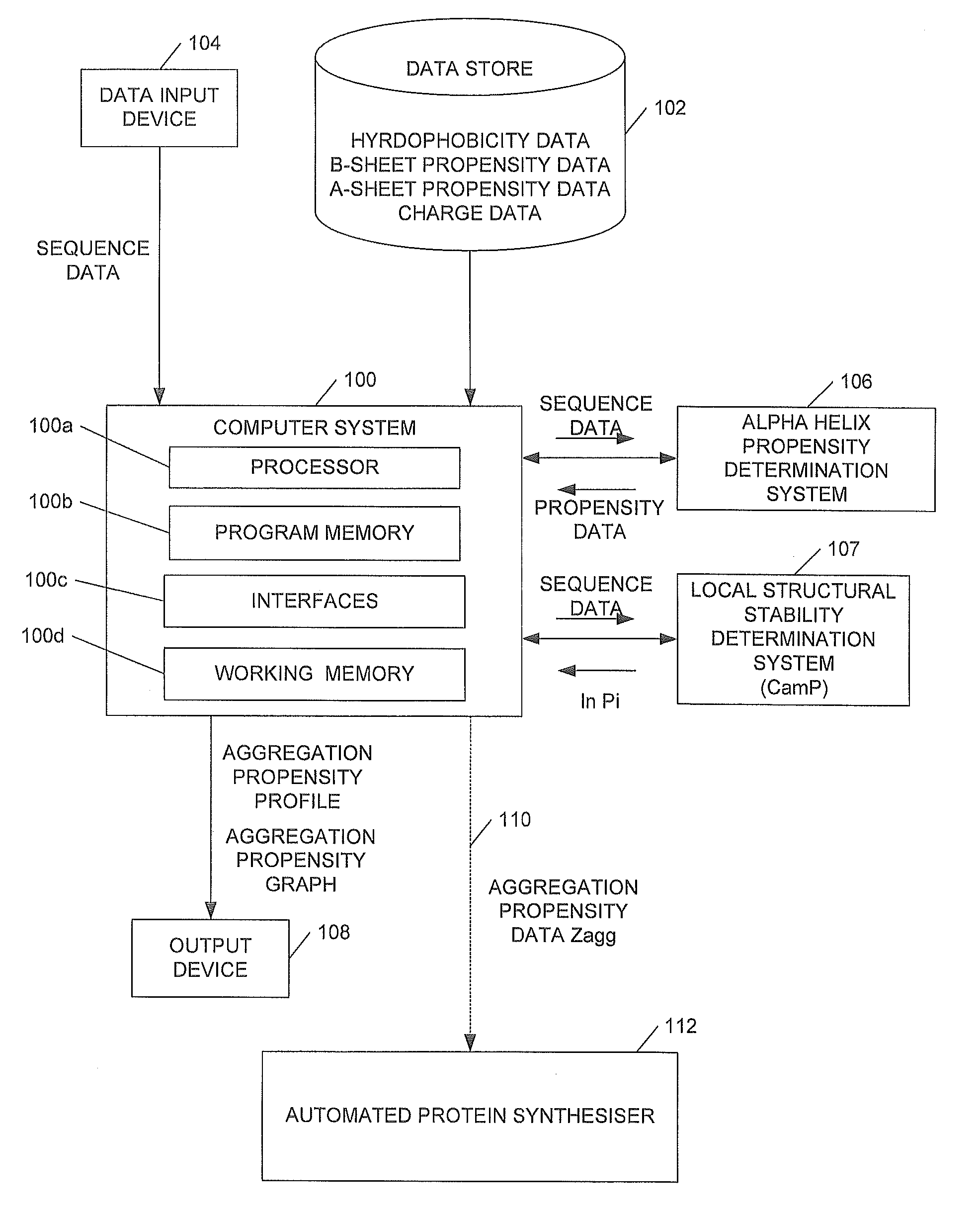
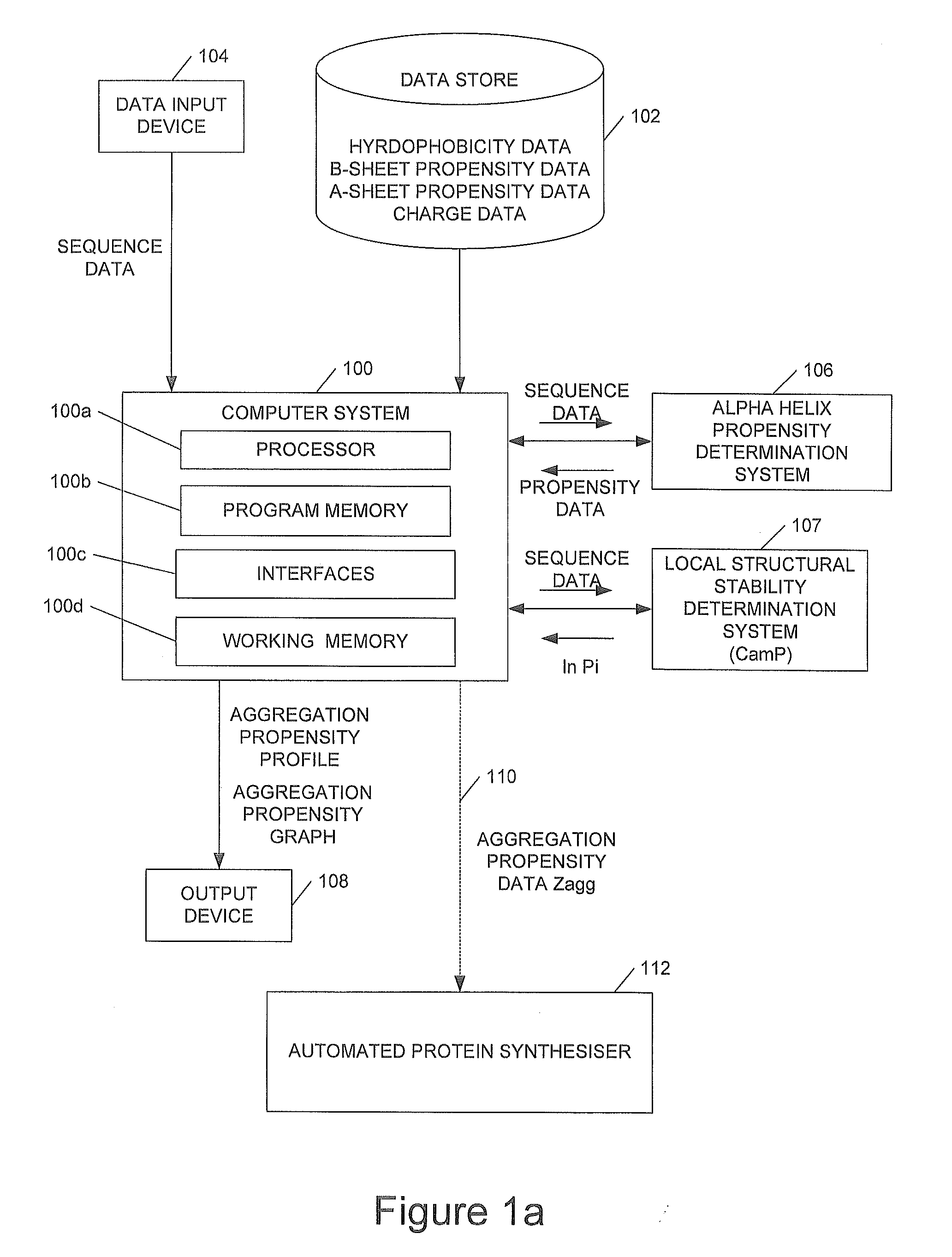
We describe methods for identifying aggregation-prone regions in structured—that is folded—proteins. Embodiments of the method use a local propensity for aggregation (Ai) at an amino acid position, this being determined by a combination of a hydrophobicity value, an α-helix propensity value, a β-sheet propensity value, a charge value and a pattern value for the amino acid position. This is combined with local structural stability values for the amino acid positions to identify one or more regions in the amino acid sequence which, in the folded protein, are predicted to promote aggregation.
Owner:CAMBRIDGE ENTERPRISE LTD
In-vitro diagnostic device and kit
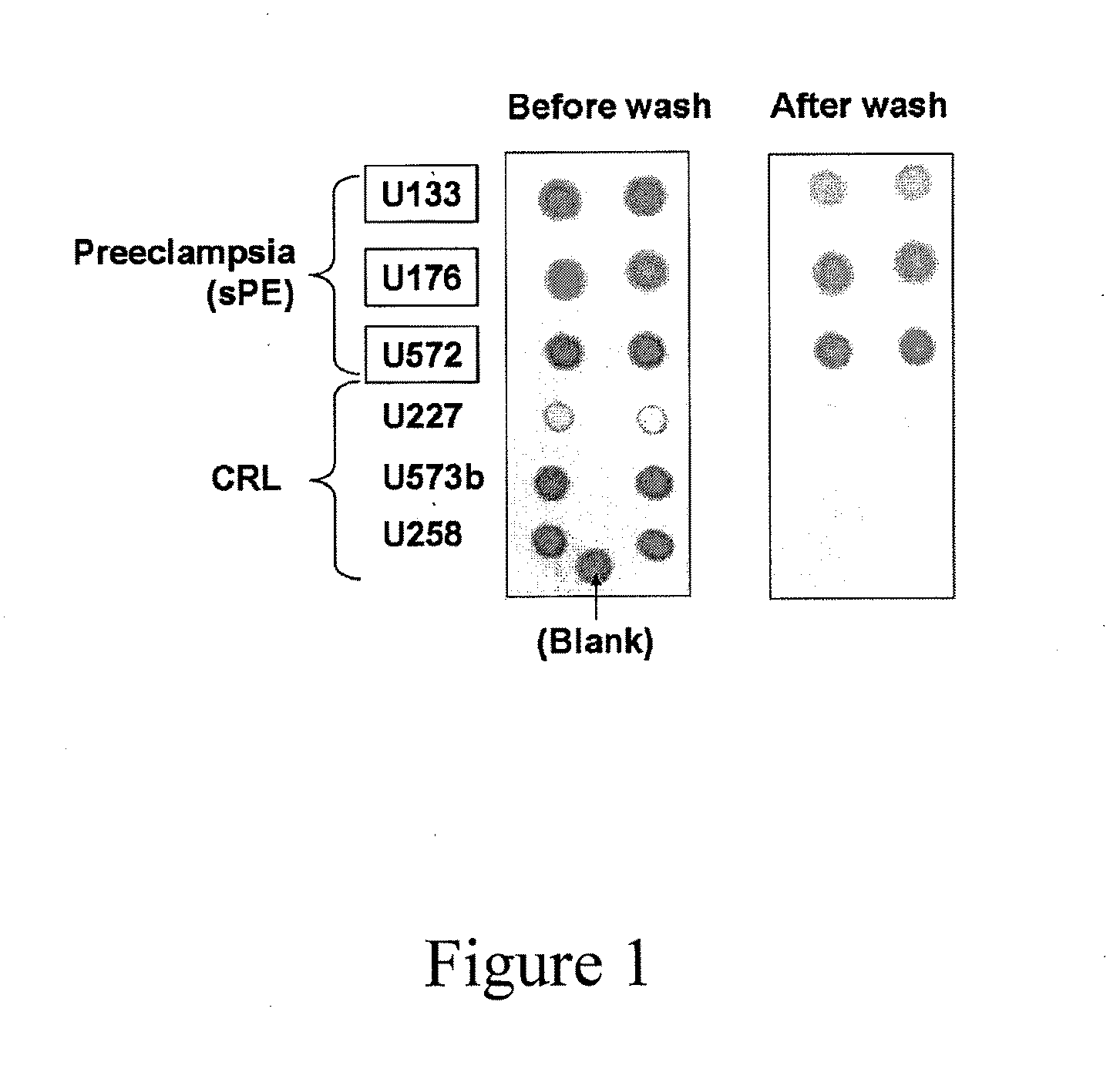
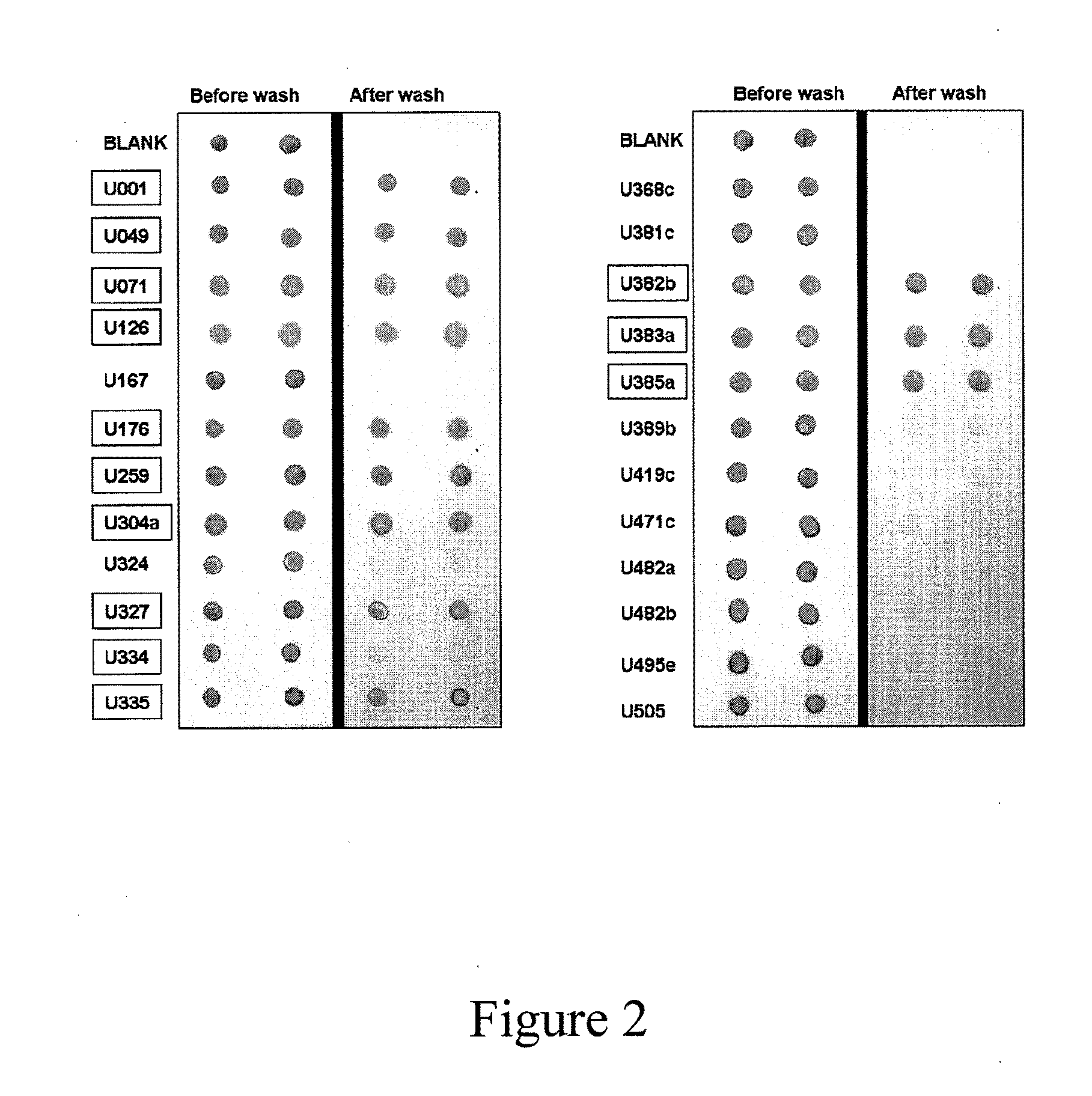
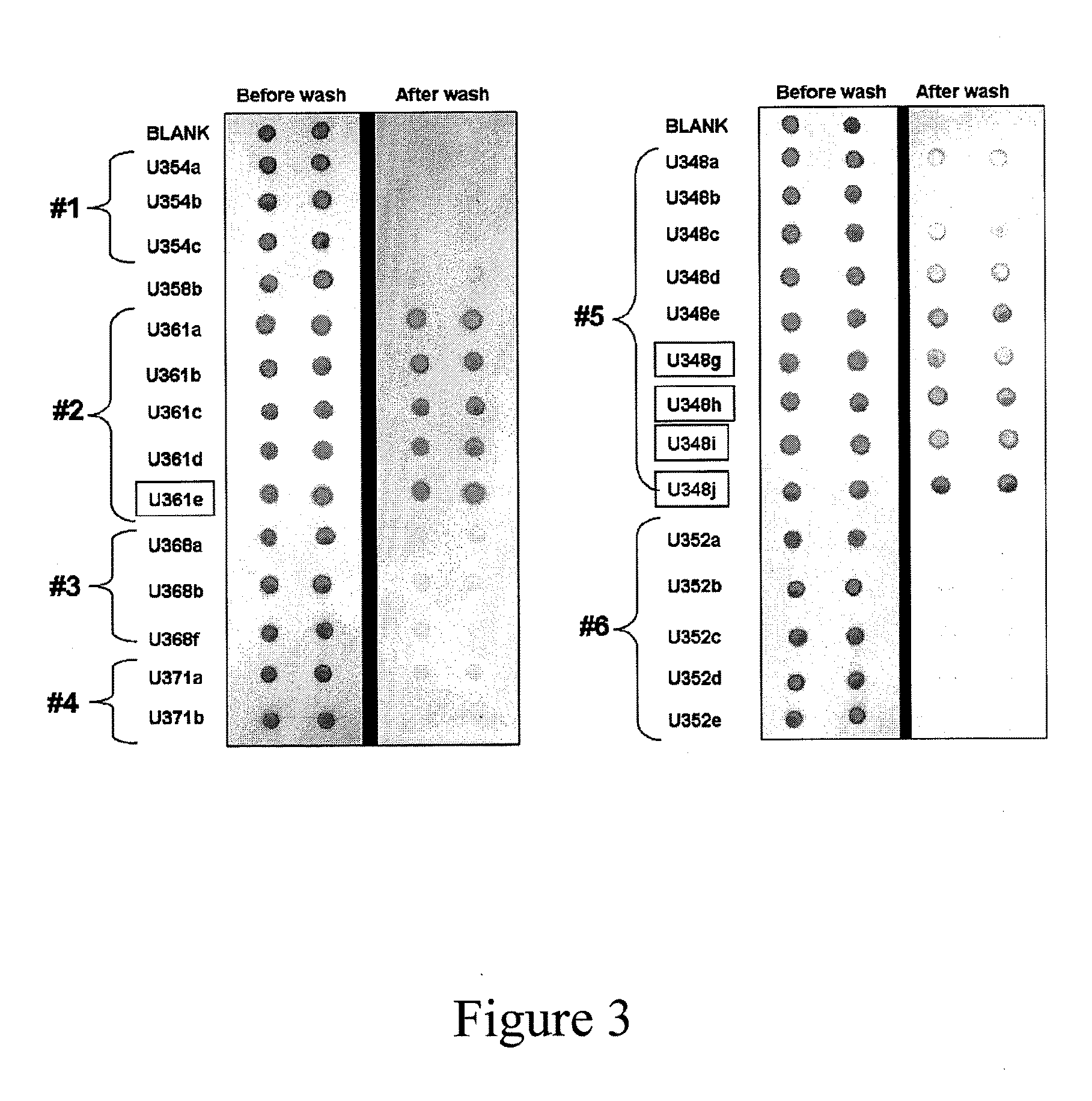
The invention provides an infiltration detection device for predicting or detecting preeclampsia. The device comprises an enclosed shell, which is formed by an upper cover and a bottom groove, wherein the upper cover and bottom groove clamp and fix each other. In the enclosed shell, the upper cover is provided with a sample feed hole. A sample to be detected and other reagents such as coloring agent, eluent, and the like can be added into the shell through the sample feed hole. In the shell, from top to bottom, at least an infiltration layer and a water absorption layer are laminated. The infiltration layer comprises a micro-porous membrane, normal proteins and other micromolecular compounds can penetrate the micro-porous membrane, but protein aggregate which is mistakenly folded cannot penetrate the micro-porous membrane. The micro-porous membrane can be made of cellulose such as cellulose nitrate or cellulose acetate. Preferably, the micro-porous membrane in the infiltration layer comprises a labeling reagent, which can be specifically bound with mistakenly-folded protein aggregate, mistakenly-folded protein aggregate, or a mixture of the labeling reagent and mistakenly-folded protein aggregate.
Owner:SHUWEN BIOTECH CO LTD
Correctly folded etanercept in high purity and excellent yield
ActiveUS20180037642A1High purityPeptide/protein ingredientsAntibody mimetics/scaffoldsPresent methodTreatment use
A mixed mode chromatography method for separating correctly folded from incorrectly folded conformations of a given protein is provided. The method is highly effective in separating correctly folded etanercept from incorrectly folded etanercept and aggregates in commercially attractive yields capable of affording etanercept preparations having very high purity in terms of correctly folded etanercept versus incorrectly folded etanercept. The invention is further directed to protein preparations and formulations comprising correctly folded proteins obtained using the present methods, and methods of treatment using the high purity preparations obtained from the mixed mode method.
Owner:COHERUS BIOSCI
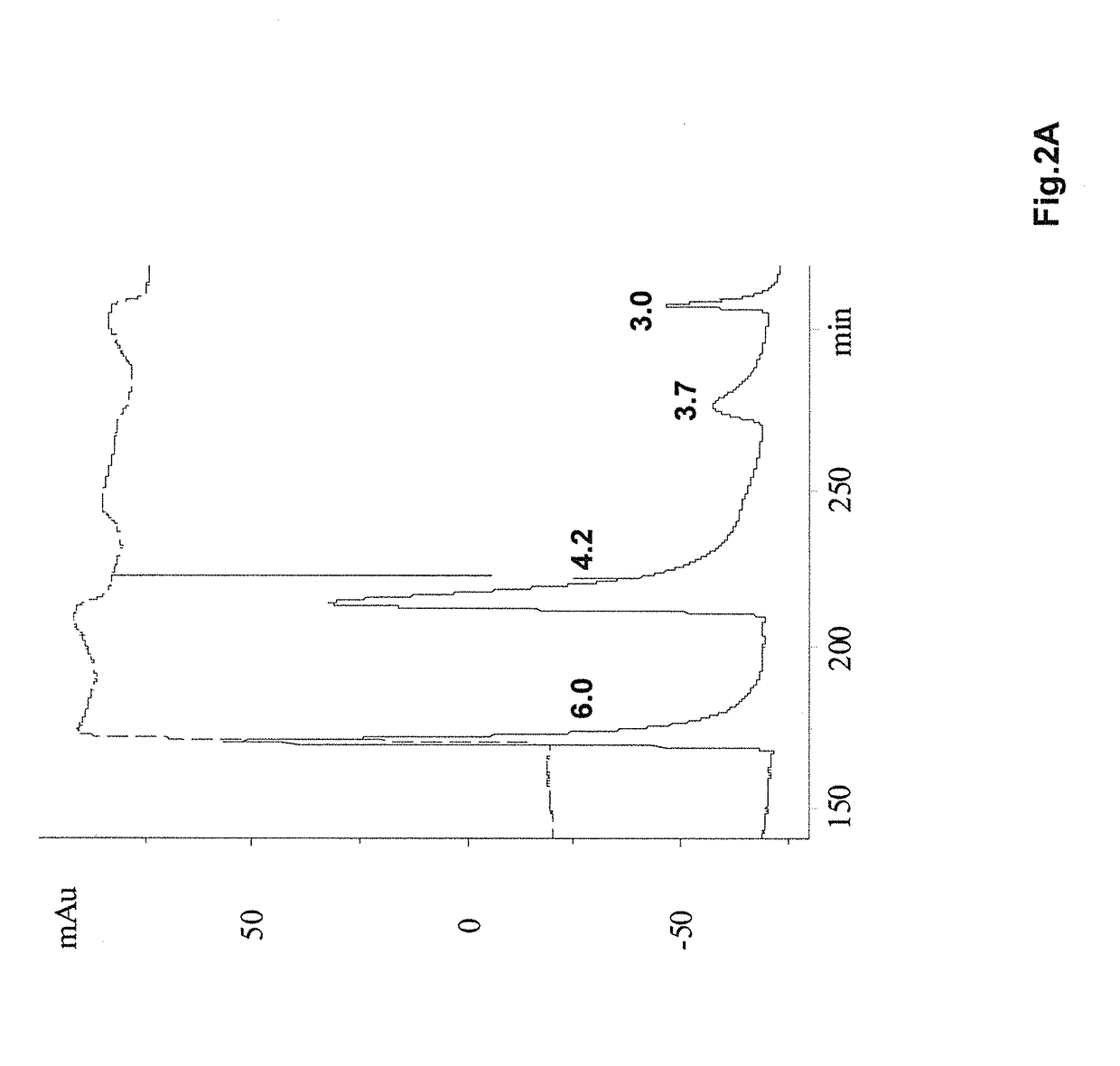
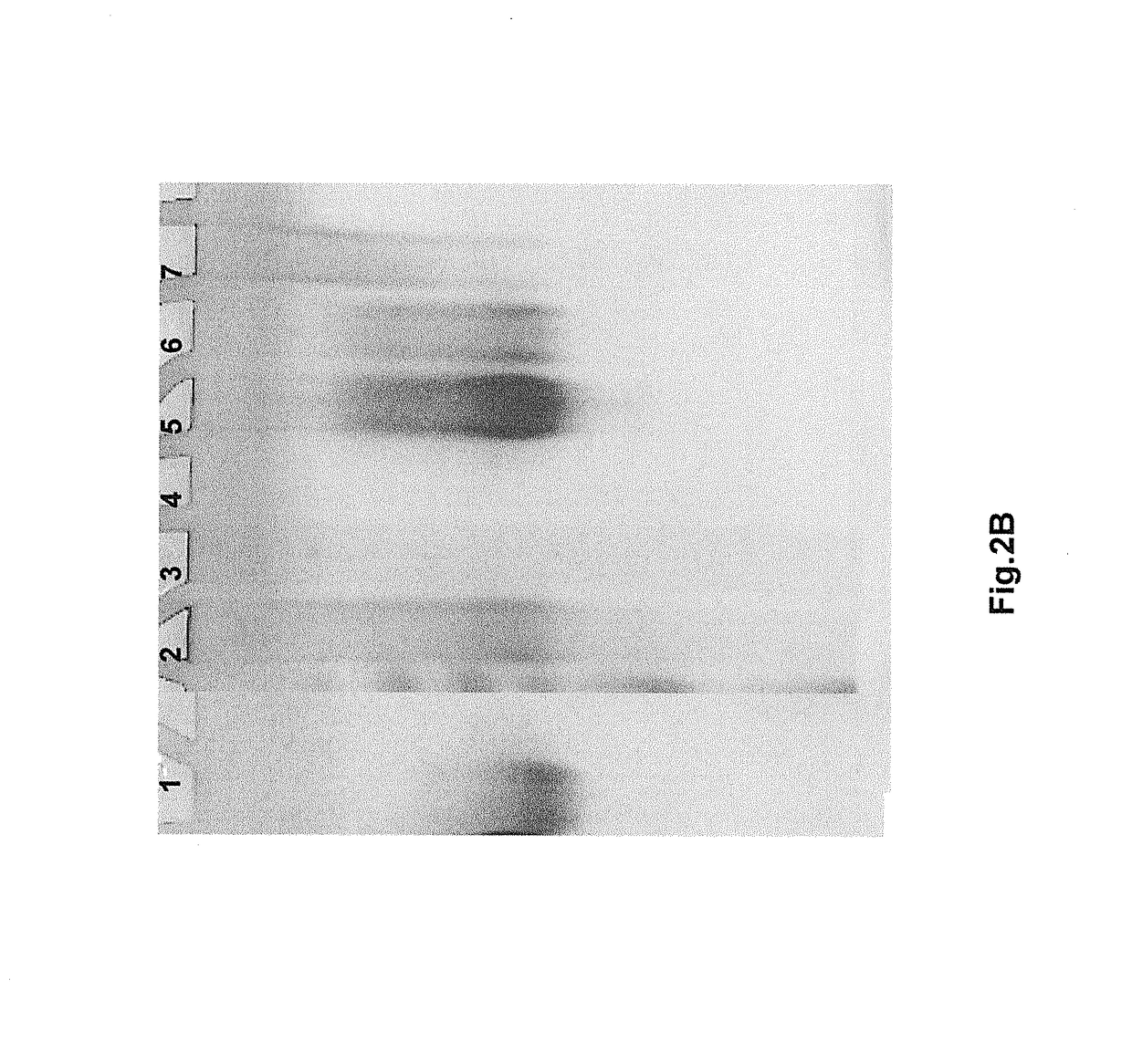
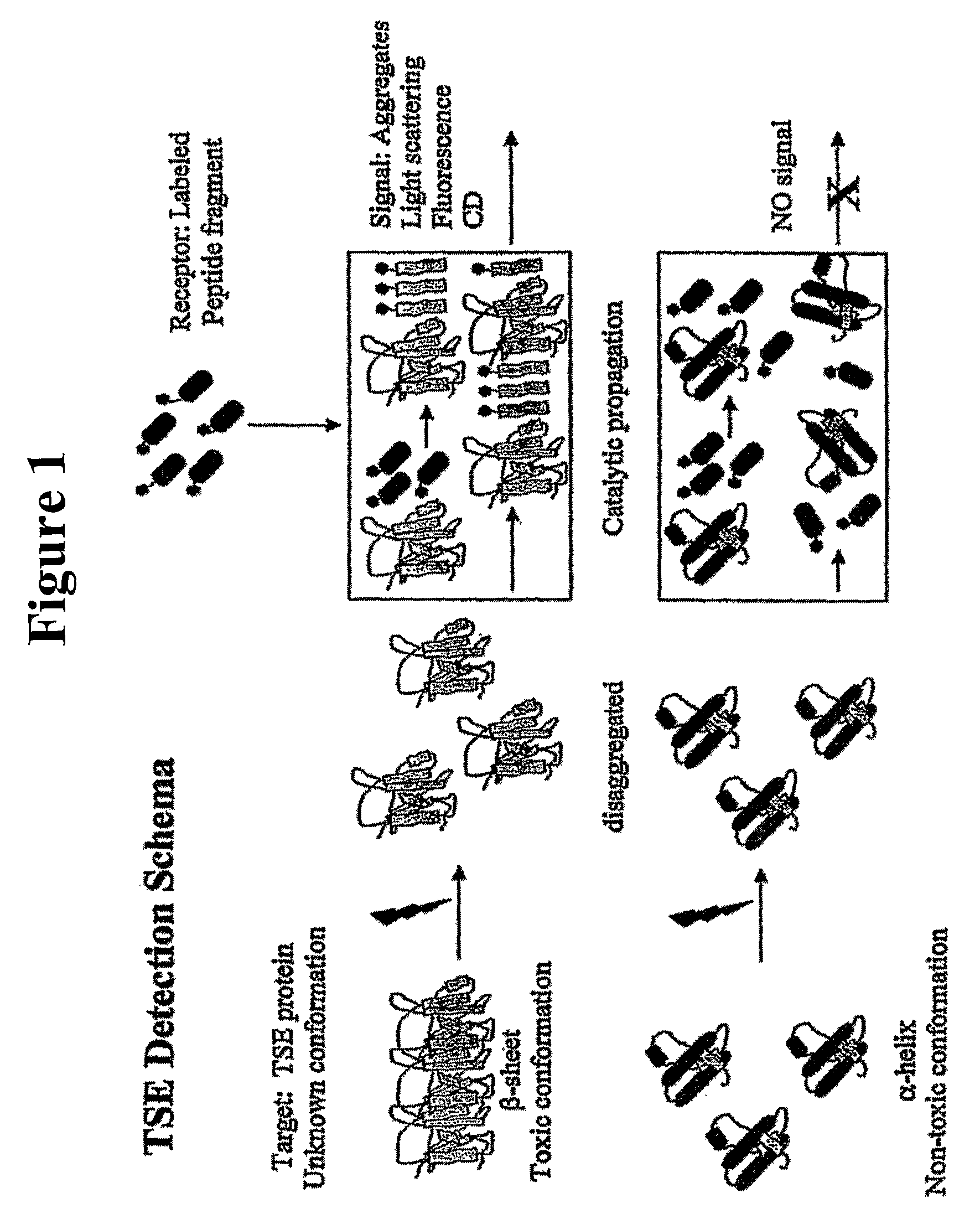
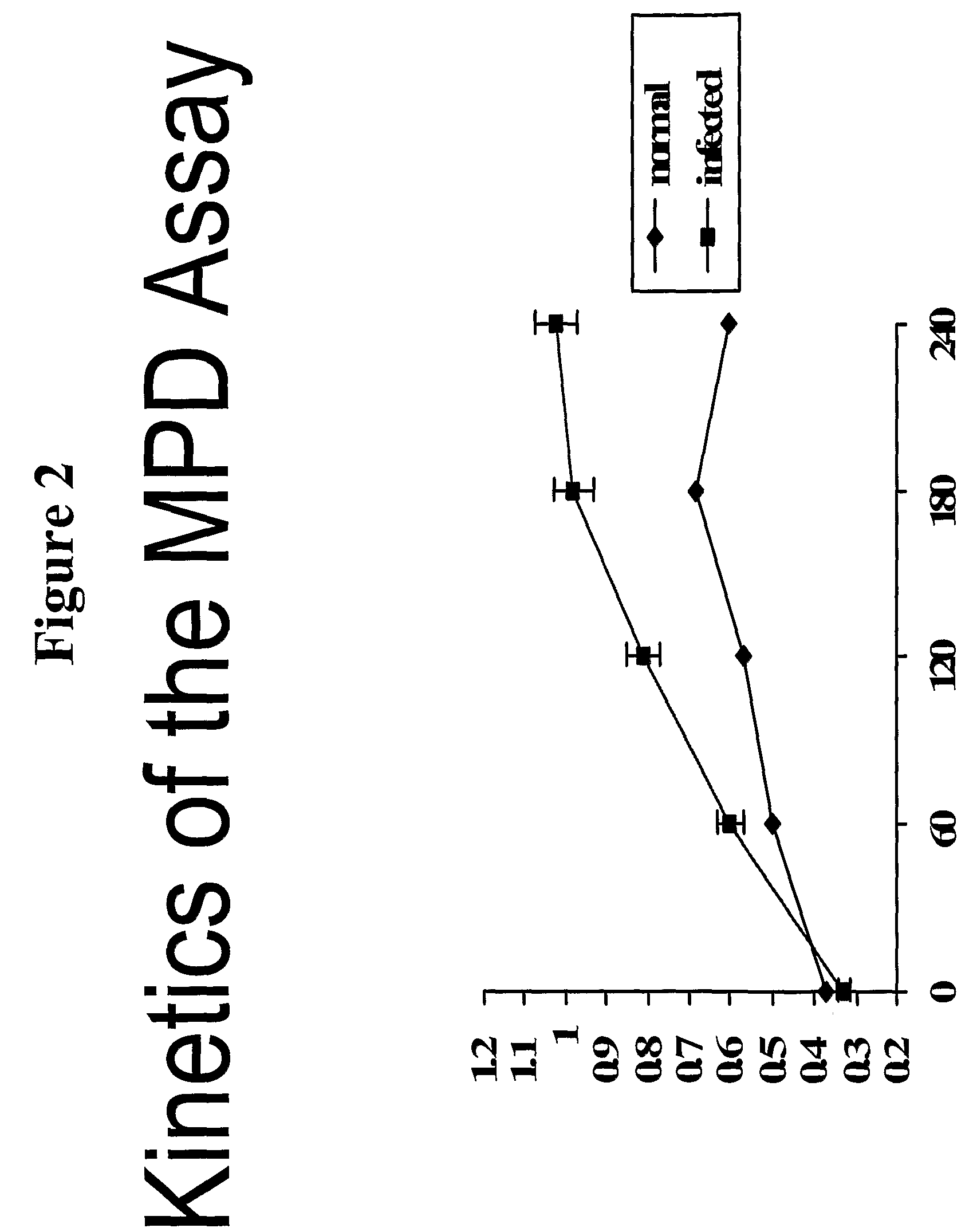
Method for detecting misfolded proteins and prions
ActiveUS8372593B2High detection sensitivityHigh sensitivityPeptide/protein ingredientsLaboratory glasswaresDiseasePeptide
The invention provides methods and kits for detecting conformationally altered proteins, such as prions or other proteins associated with disease states, in a sample. The methods comprise selectively capturing and separating complexes of peptide and conformationally altered protein from substances that interfere with detection of such complexes, and preferably amplification of the detection signal b addition of a second double-labeled peptide.
Owner:PRESYMPTO INC
Protein Production Method
ActiveUS20140296489A1Good yieldIncrease temperaturePeptide/protein ingredientsAntibody mimetics/scaffoldsTiterChemistry
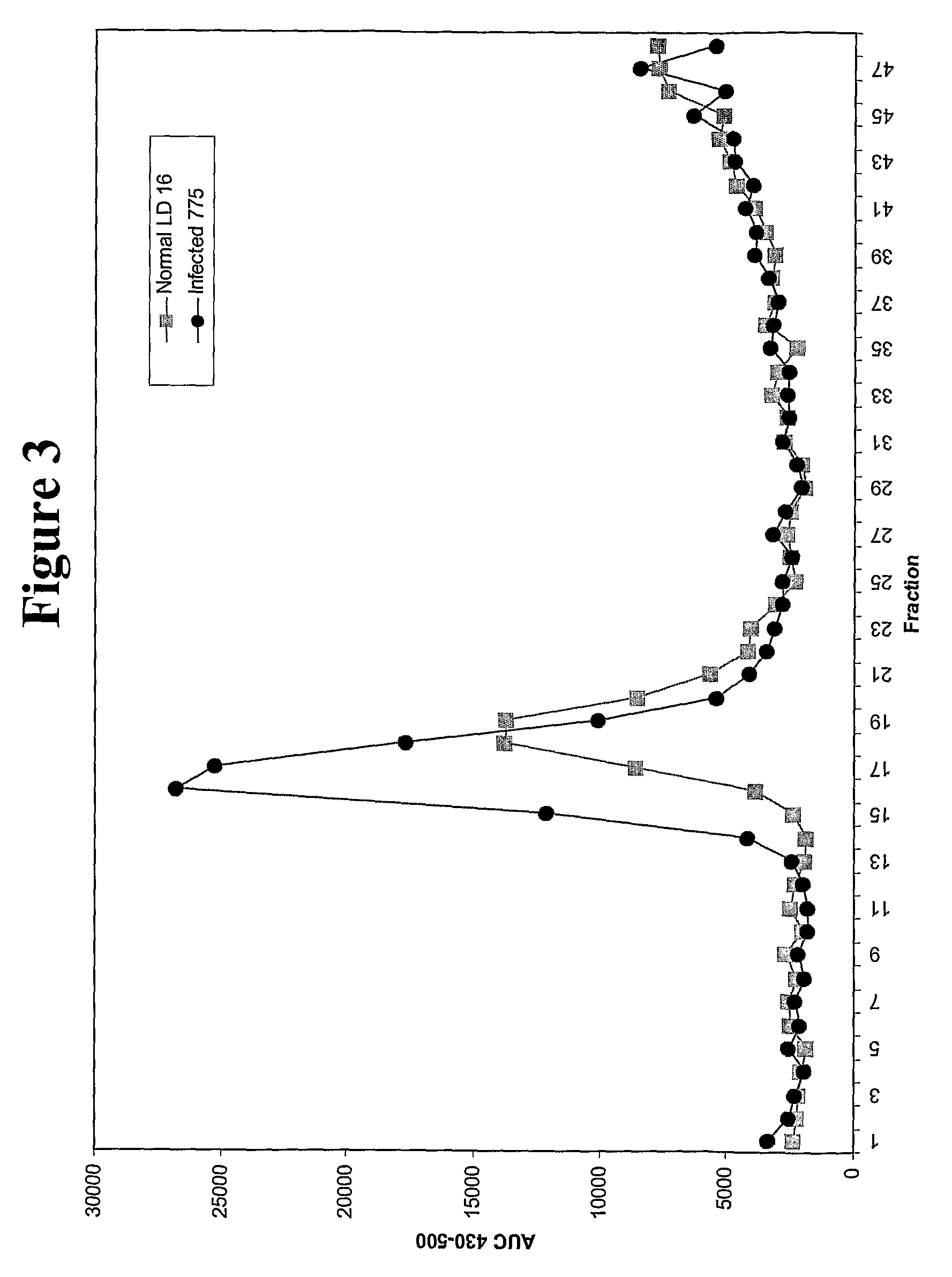
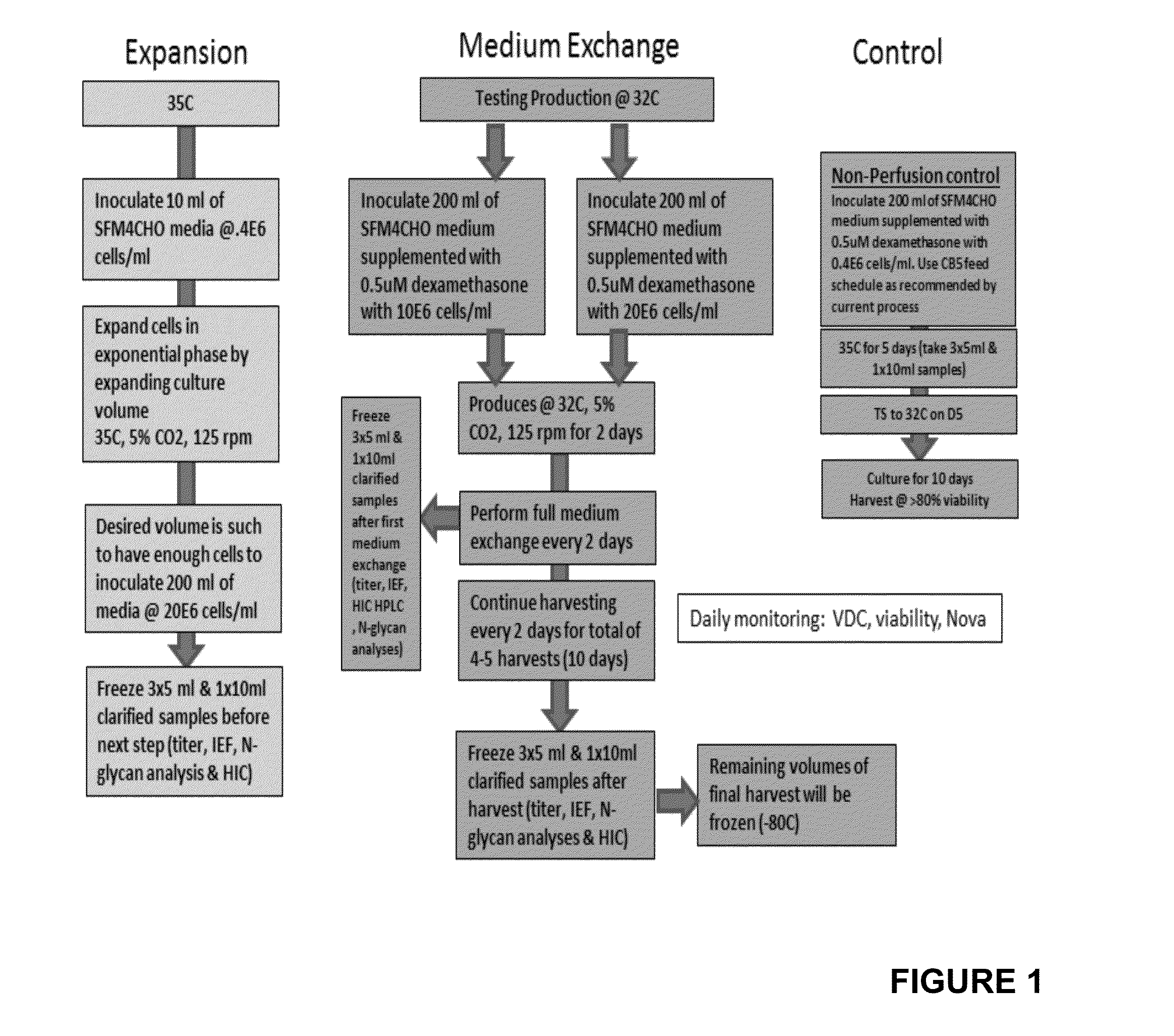
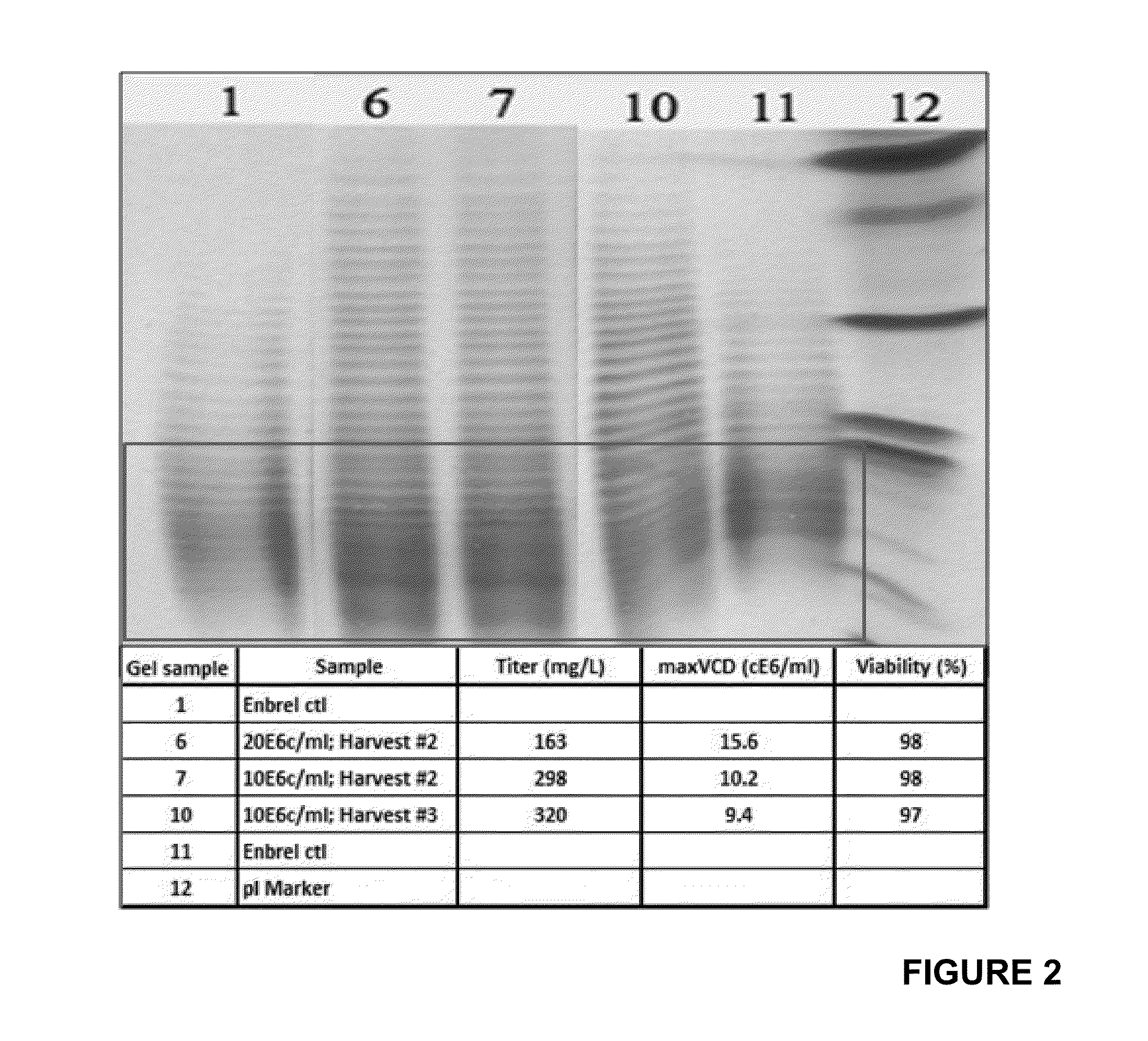
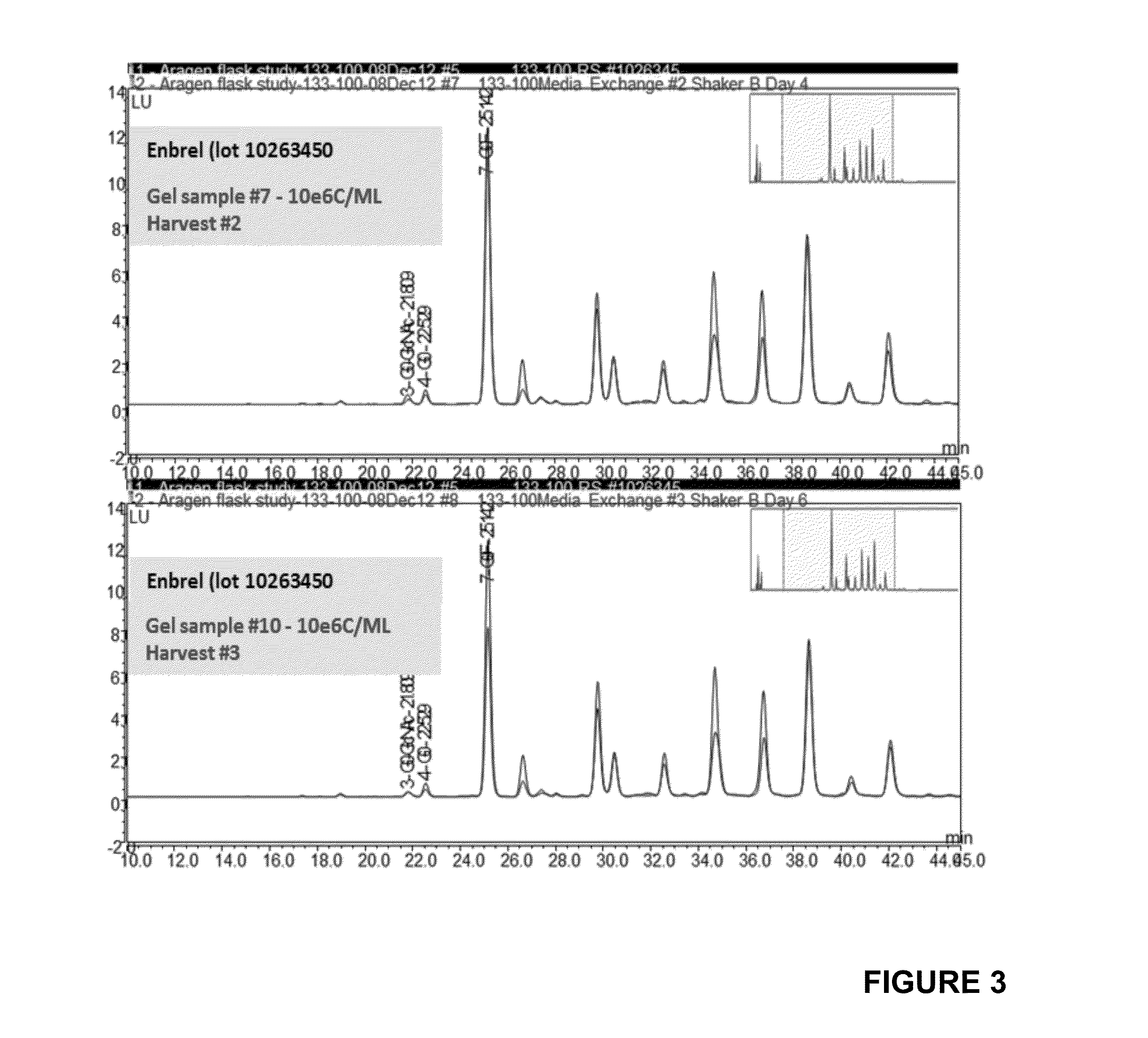
Production of etanercept using perfusion methods achieves attractive yields of properly folded protein. Desired temperature, feed media, titers and percent correctly folded protein are disclosed.
Owner:COHERUS BIOSCI
Methods of renaturation of recombinant proteins
InactiveUS20110034678A1High protein concentrationPeptide preparation methodsDepsipeptidesSulfateChromatography column
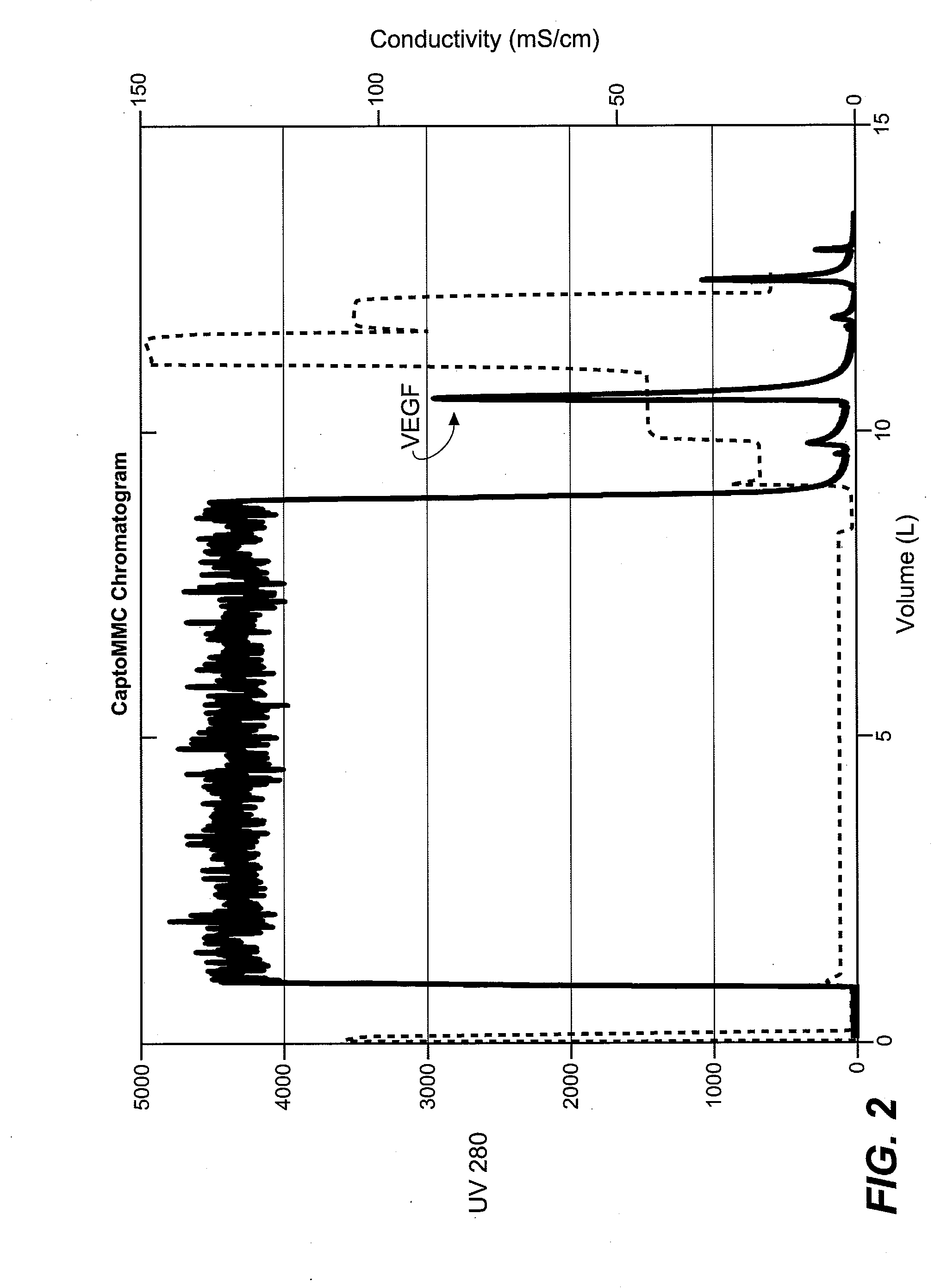
The present invention provides large-scale methods for renaturation of proteins comprising adding a solution of denatured, chemically modified or reduced proteins to a refolding buffer containing sulfate derived from H2SO4 and / or MgSO4 in the presence of guanidine. The present invention further provides methods of isolating a refolded protein at a concentration of about 0.4 to 3.0 gm / L by using a hydrophobic interaction chromatography (HIC) column.
Owner:AEROVANCE INC
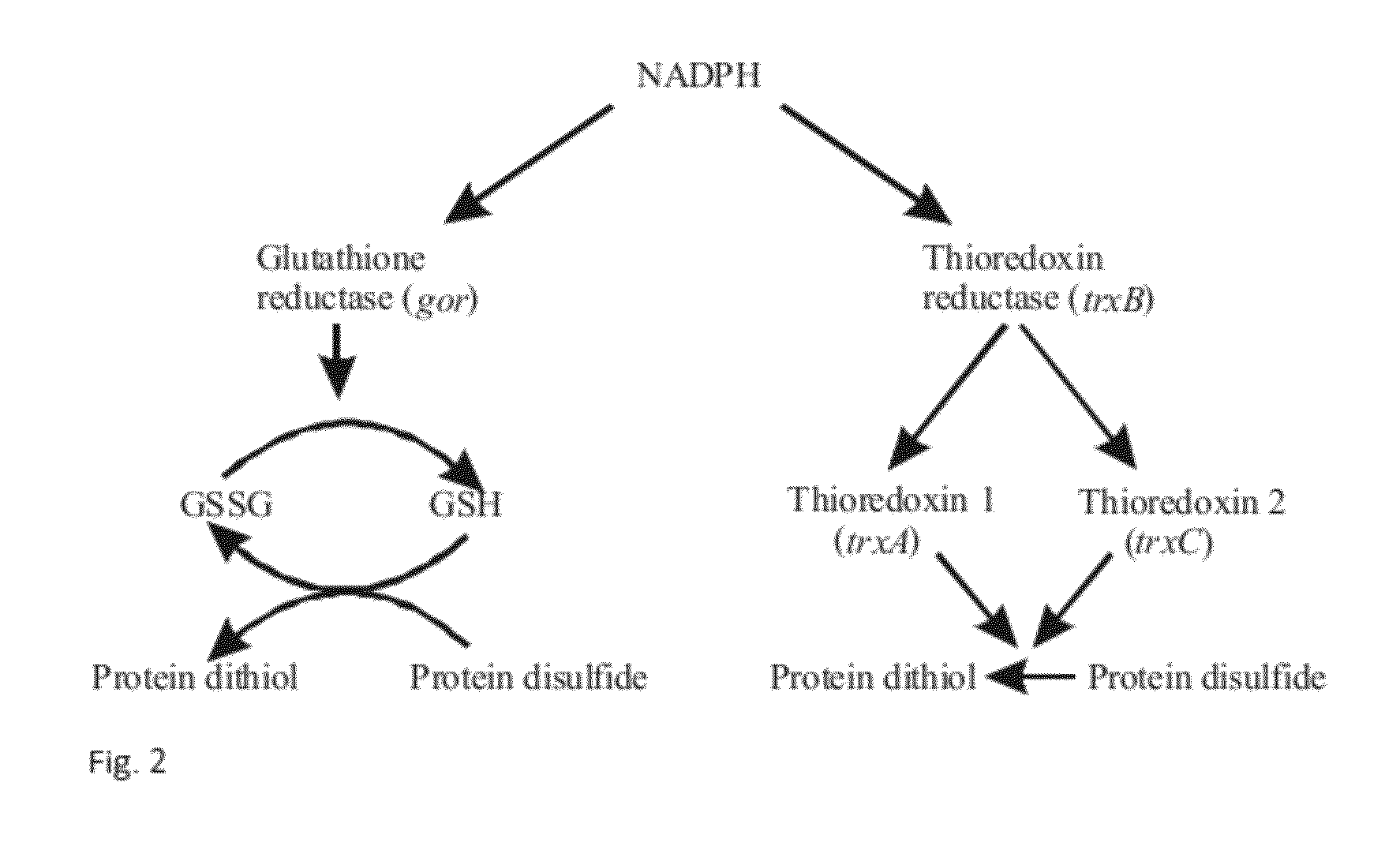
Method for Producing Natively Folded Proteins in a Prokaryotic Host
The present invention relates to a method for producing a protein of interest containing one or more disulfide bonds in its native state. The method comprises that a prokaryotic host cell is genetically engineered to express the protein of interest and a sulfhydryl oxidase in the cytoplasm of the host cell. The protein of interest is formed in a soluble form and contains disulfide bonds due to the presence of the sulfhydryl oxidase in the cytoplasm of said host cell. The present invention relates also to a prokaryotic host cell and a vector system for producing a protein of interest containing natively folded disulfide bonds.
Owner:UNIV OF OULU
Genes encoding for genetic stability, gene expression and folding proteins
InactiveUS20050009152A1High expressionIncrease productionSugar derivativesBacteriaMicroorganismGene expression
The invention relates to novel nucleic acid molecules, to the use thereof for constructing genetically improved microorganisms and to methods for preparing fine chemicals, in particular amino acids, with the aid of said genetically improved microorganisms.
Owner:DAESANG
Methods and kits for detecting misfolded proteins
Methods, kits and compounds are provided that relate to the diagnosis, treatment, and / or prevention of preeclampsia.
Owner:YALE UNIV
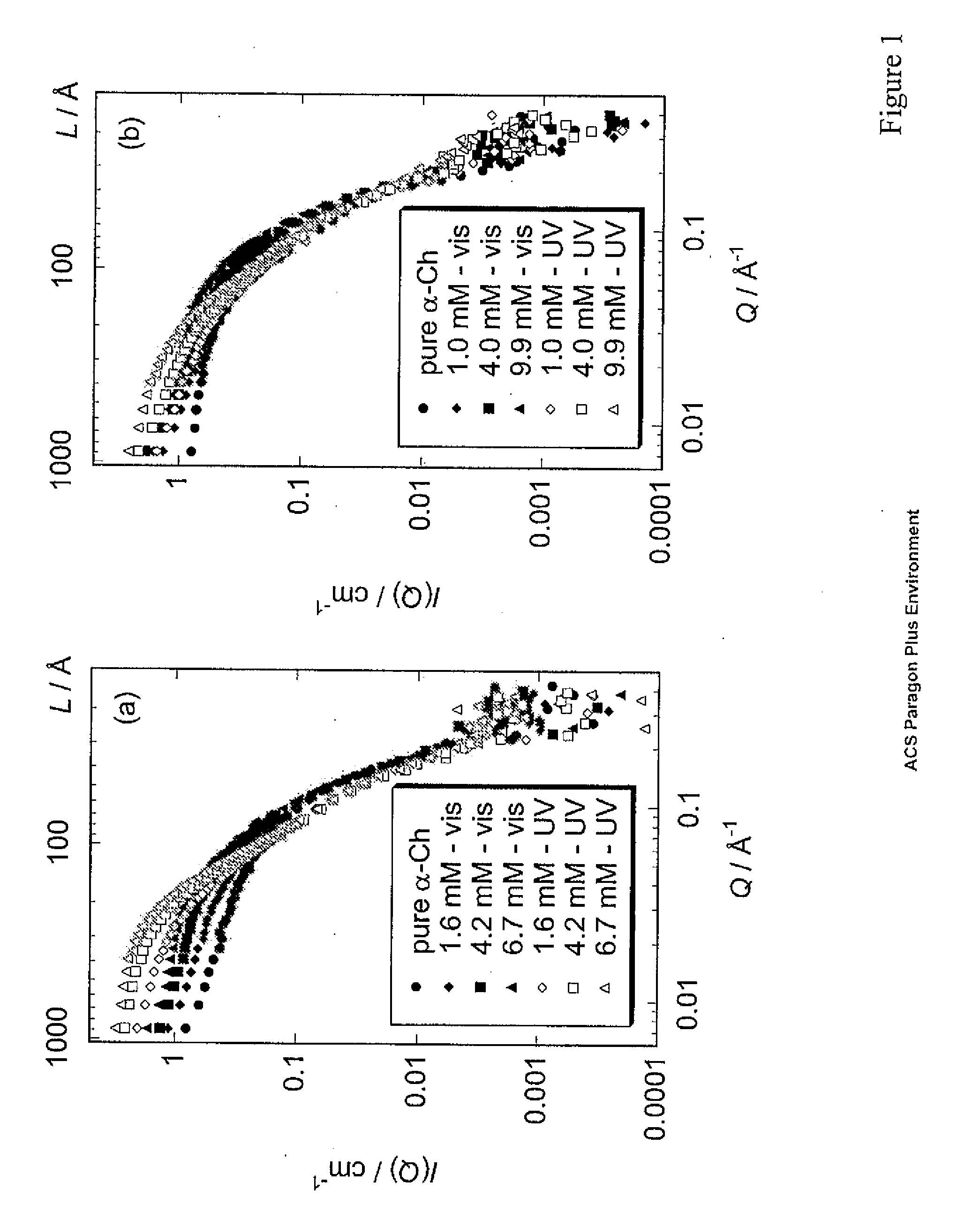
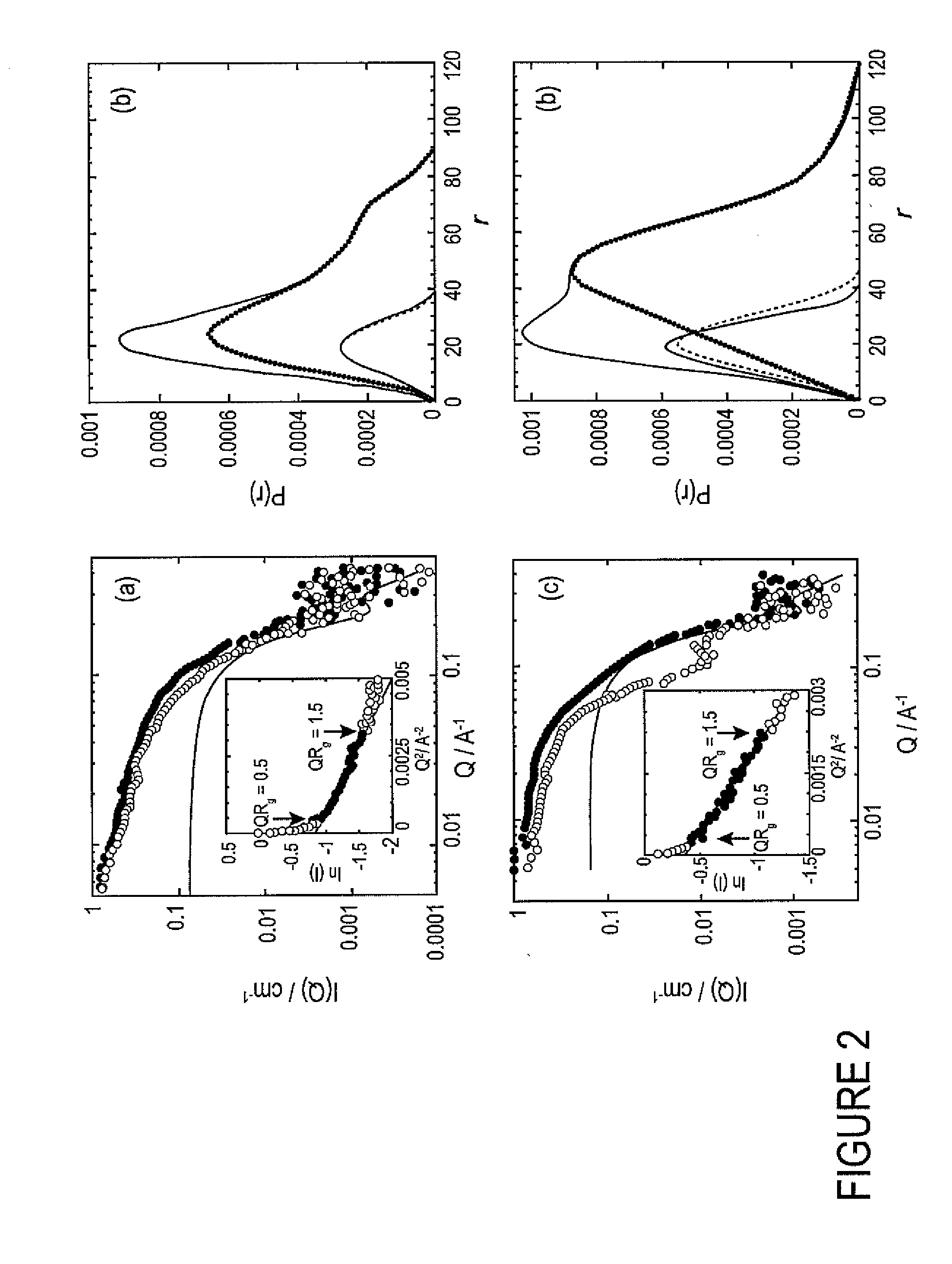
Using photo-responsive surfactants to reversibly control protein aggregation with light illumination
InactiveUS20090075318A1Microbiological testing/measurementPeptide preparation methodsCrystallographyCell Aggregations
The present invention relates to methods of inducing protein folding using light illumination. More specifically the invention relates to shape-reconstruction analysis applied to small angle neutron scattering (SANS) data that is used to determine the structure of partially-folded proteins in non-native conformations and supramolecular complexes undergoing self- or hetero-association in solution as a result of partial unfolding with a photoresponsive surfactant.
Owner:UNIV OF SOUTHERN CALIFORNIA
Method for synthesizing graphene-protein composite material
InactiveCN108743417ARepair damageGuaranteed cleanlinessCosmetic preparationsHair cosmeticsCuticleReaction temperature
The invention discloses a method for synthesizing a graphene-protein composite material and belongs to the field of cosmetics. The method comprises the following steps: carrying out ultrasonic dispersion on graphene oxide into DMF (dimethyl formamide), preparing a 1-20mg / mL solution, adding a mercapto compound in the presence of nitrogen, stirring to react for 0.5-12 hours, adding a reducing agent, carrying out a reduction reaction at 45-200 DEG C for 0.5-120 hours, centrifuging for 3-5 times, and washing with water to remove an unreacted mercapto compound and obtain mercapto graphene; dissolving keratin with water to obtain a protein solution, putting a denaturant into the solution, folding proteins to expose mercapto, and forming a protein degeneration solution; putting the mercapto graphene into the protein degeneration solution, and crosslinking and assembling graphene with proteins through generated disulfide bonds, thereby obtaining the graphene-protein composite material. By virtue of a super large specific surface area of graphene, a network structure can be formed to moisture hair. With recombination of the proteins, damaged hair cuticles can be effectively repaired, finesundries can be sucked, and hair cleanliness can be maintained.
Owner:山东利特纳米技术有限公司
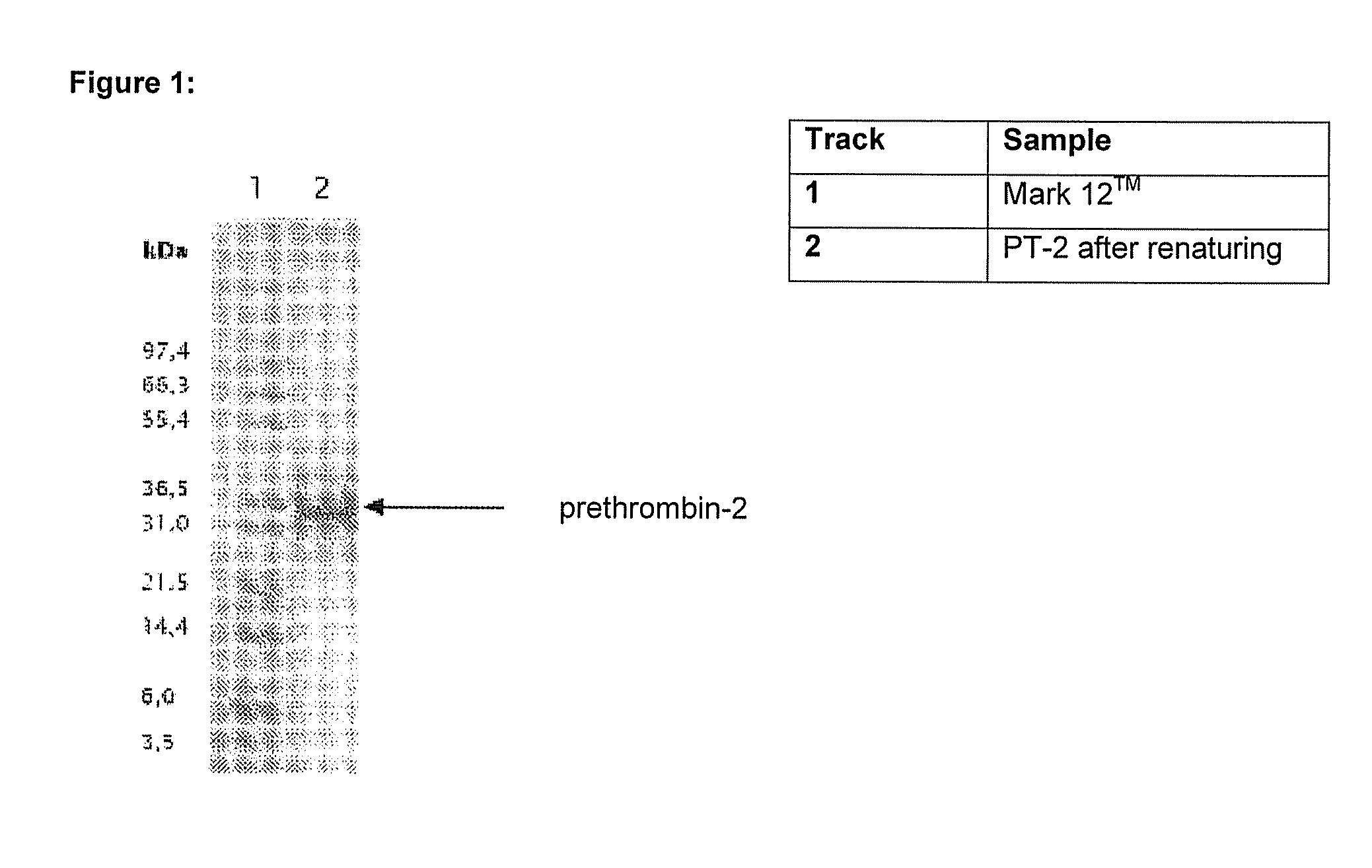
Method for producing recombinant thrombin
The invention relates to a method for producing folded prethrombin, wherein inclusion bodies, which contain unfolded prethrombin or a derivative thereof, are solubilized in a solubilization buffer containing at least one chaotropic compound and at least one organic disulfide compound. The invention further relates to methods for producing thrombin and a-thrombin and derivatives thereof. The invention also relates to solutions that contain folded proteins, which can be produced by the methods according to the invention.
Owner:WACKER CHEM GMBH
Methods for structural analysis of proteins
InactiveUS20090131675A1Overcome limitationsOrganic chemistryBiological testingCysteine thiolateBinding site
The invention provides methods and compositions for protein structure analysis, including substrate binding sites, sites of protein-protein interactions, three dimensional structure analysis, and stability, all with single amino acid resolution. In general, the subject methods involve introduction of cysteine residues, which serve as probes for physical analysis, into a protein by translational misincorporation in vivo. In many embodiments, proteins containing misincorporated cysteine residues are reacted with a crosslinking agent that covalently links misincorporated cysteine residues to a proximal amino acid in the folded protein. These methods, termed “MXLINK” methods, may be used for protein tertiary structure analysis. In other embodiments, cysteine-misincorporated proteins are used in protein footprinting methods, termed “MPAX” or “MSX” methods.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Renaturation method of insulin glargine precursor
ActiveCN103694339ASimple purification processShort reaction timePeptide preparation methodsInsulinsInsulin glargineCombinatorial chemistry
The invention discloses a renaturation method of an insulin glargine precursor, and belongs to the field of biomedical protein folding. The method comprises the following steps: dissolving the insulin glargine precursor in a modifier solution, adding a reducer for reduction, adjusting the pH value to be 9.5-11.5, controlling the temperature of a reaction system to be 35-45 DEG C, and reacting for 30-60 minutes to obtain a modified insulin glargine precursor solution; adding the modified insulin glargine precursor solution into a dilute buffer solution, adding a protein folding additive, adjusting the pH value to be 9.5-11.5, continuously inletting air into the solution, controlling the temperature of the reaction system to be 0-20 DEG C, and reacting for 2-40 hours to obtain an insulin glargine renaturation solution. According to the method, the renaturation reaction time is shortened, the correctly folded protein content is increased, the renaturation efficiency is improved to be 51%-62%, the production cost is reduced, and the large-scale industrialization production and application are facilitated.
Owner:ZHUHAI UNITED LAB
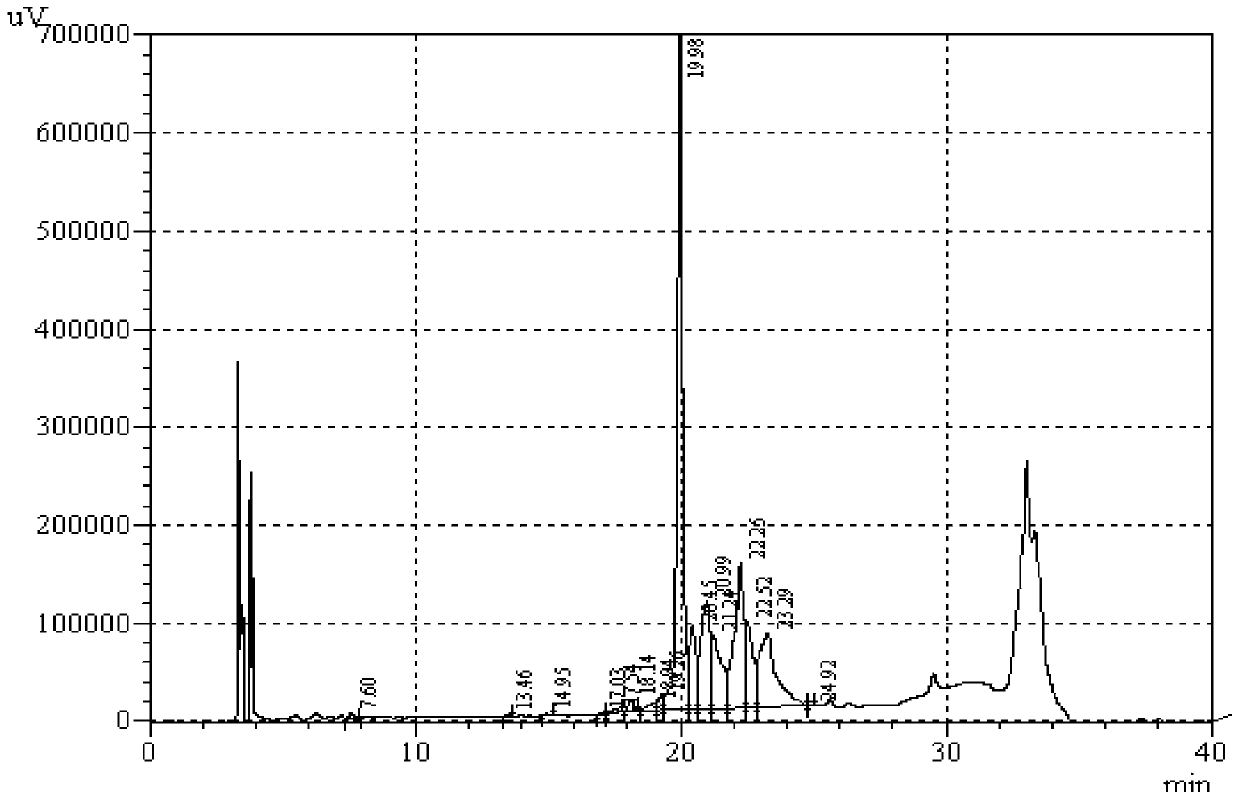
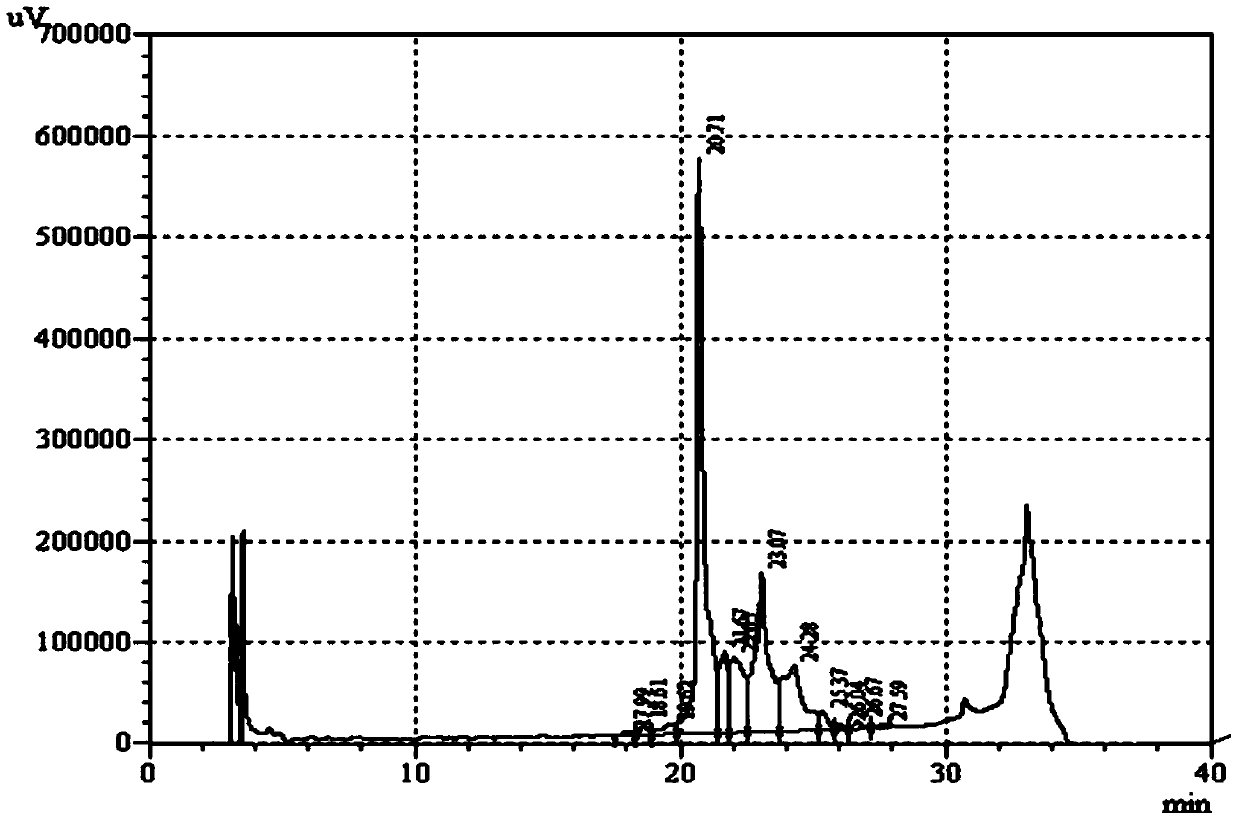
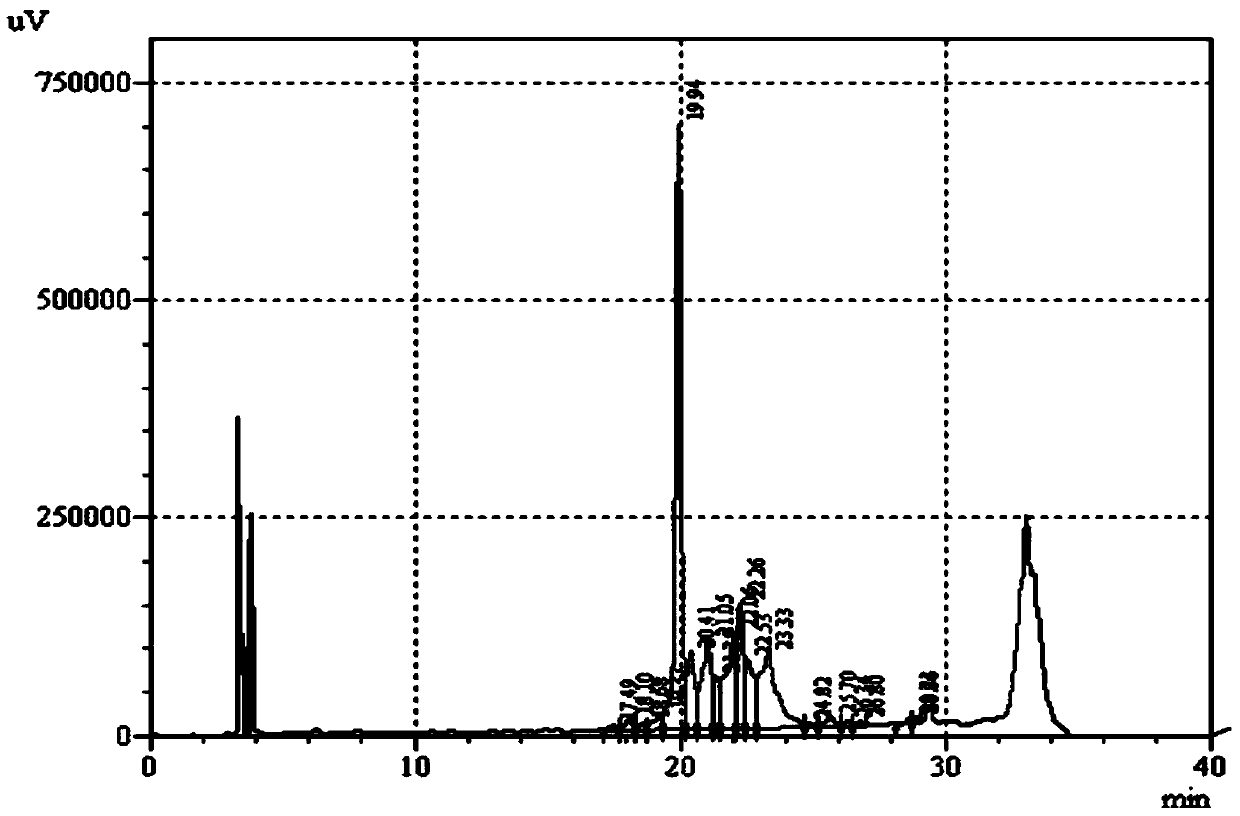
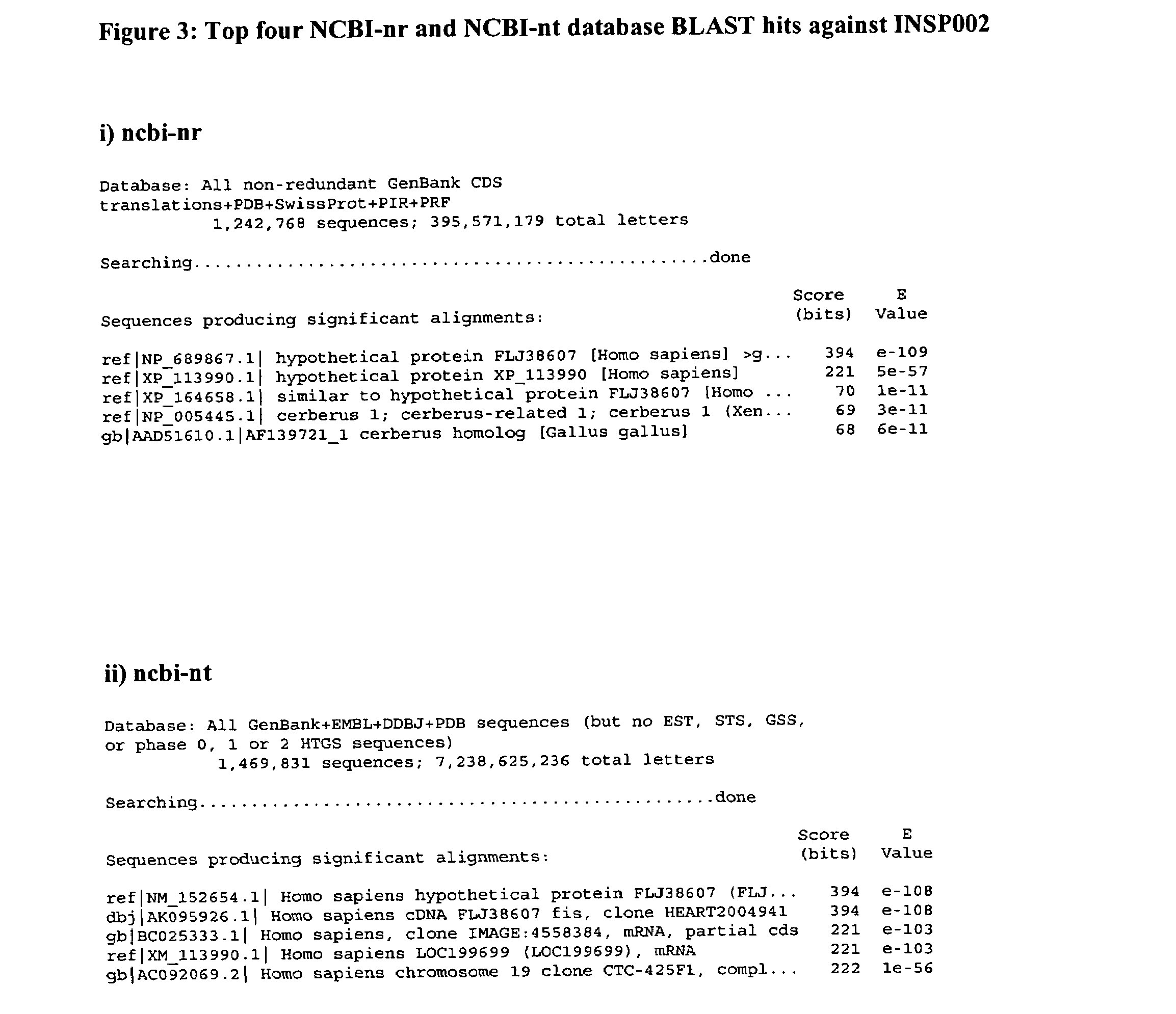
Cystine-knot fold protein
This invention relates to a novel protein (INSP002), herein identified as a secreted protein that is a member of the Dan family of the cystine-knot fold cytokine superfamily and to the use of this protein and nucleic acid sequences from the encoding genes in the diagnosis, prevention and treatment of disease.
Owner:ARES TRADING SA
Affinity regions
InactiveCN101421304AEliminate accumulationRelieve symptomsNervous disorderImmunoglobulins against animals/humansEpitopeBiology
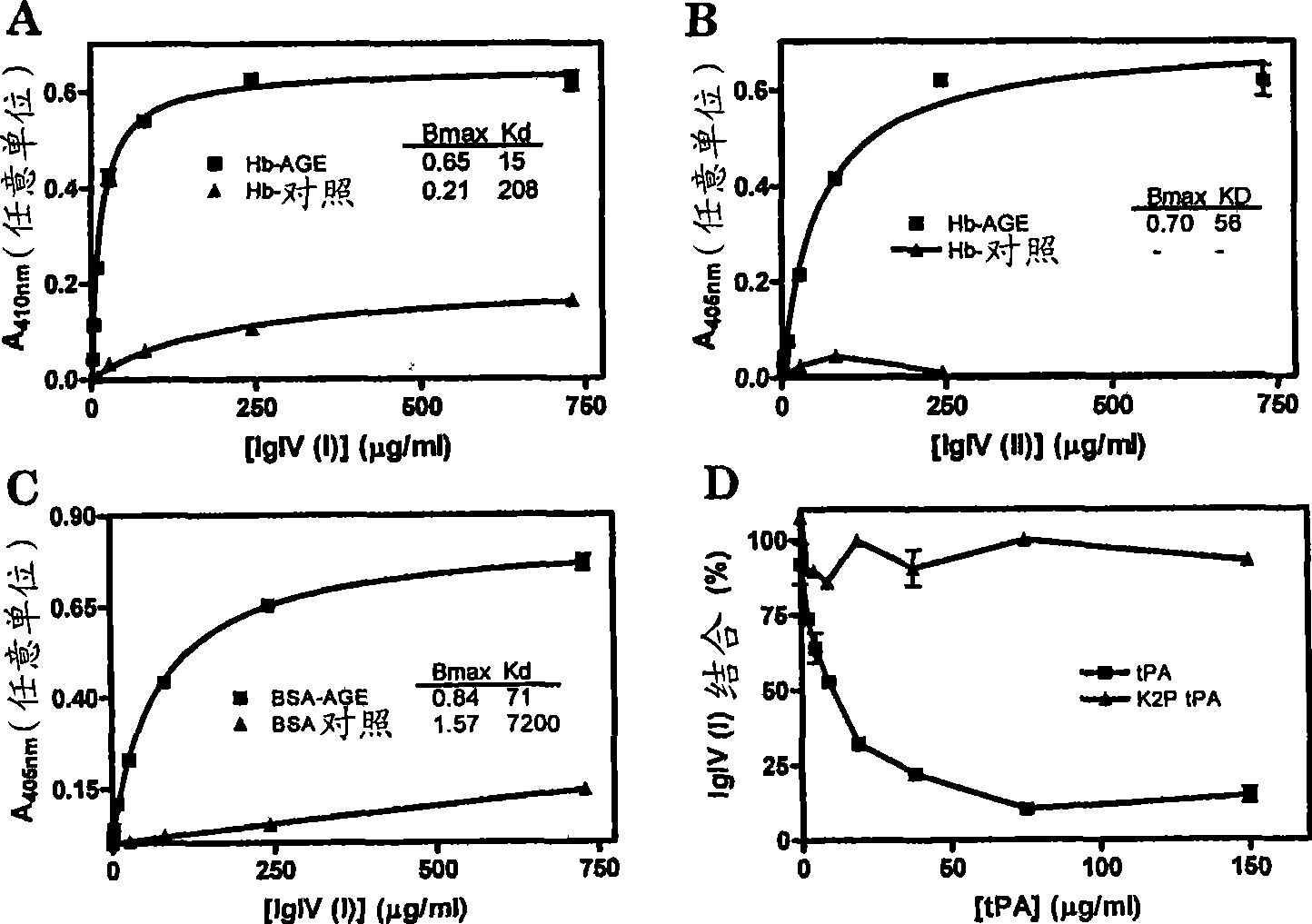
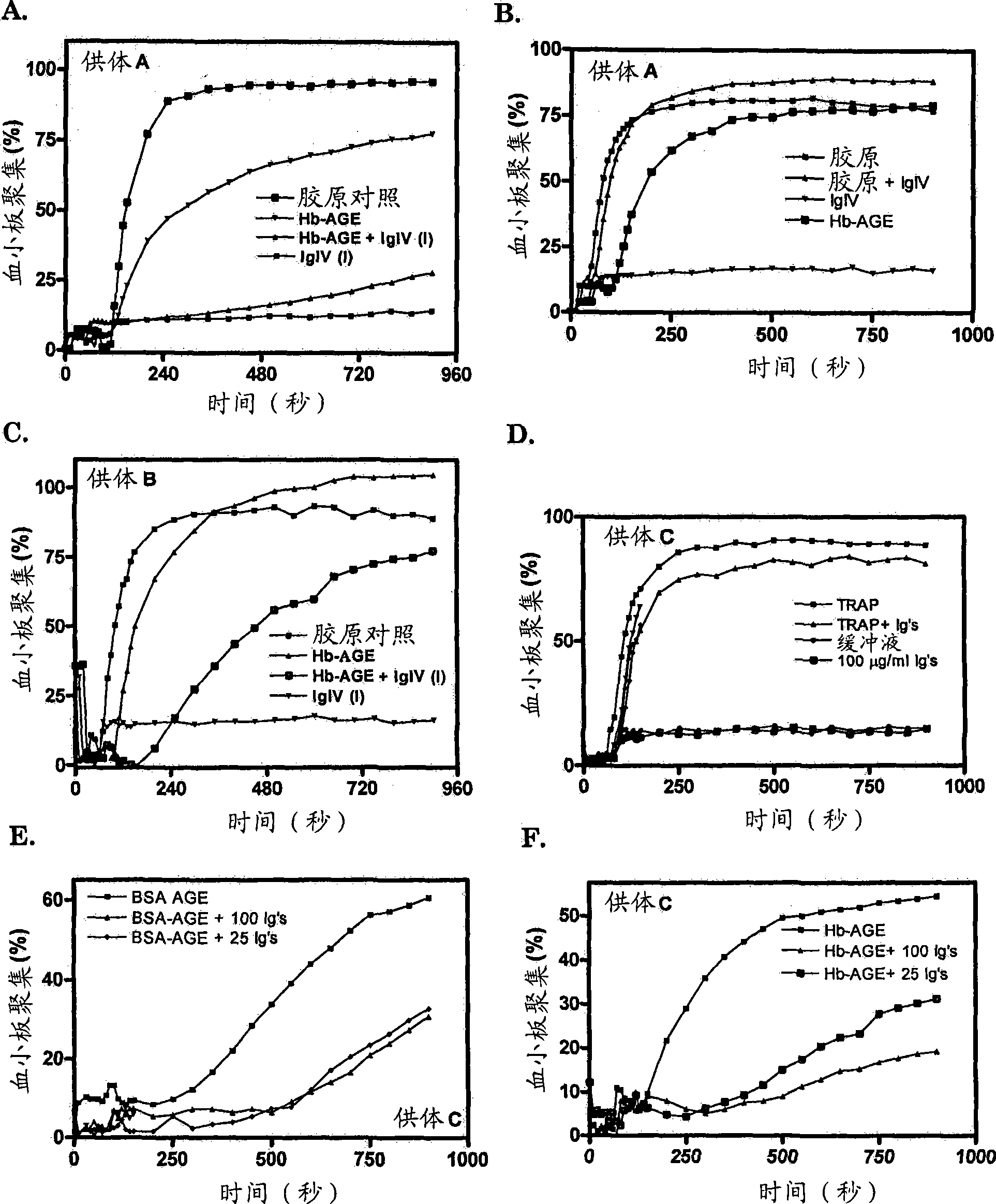
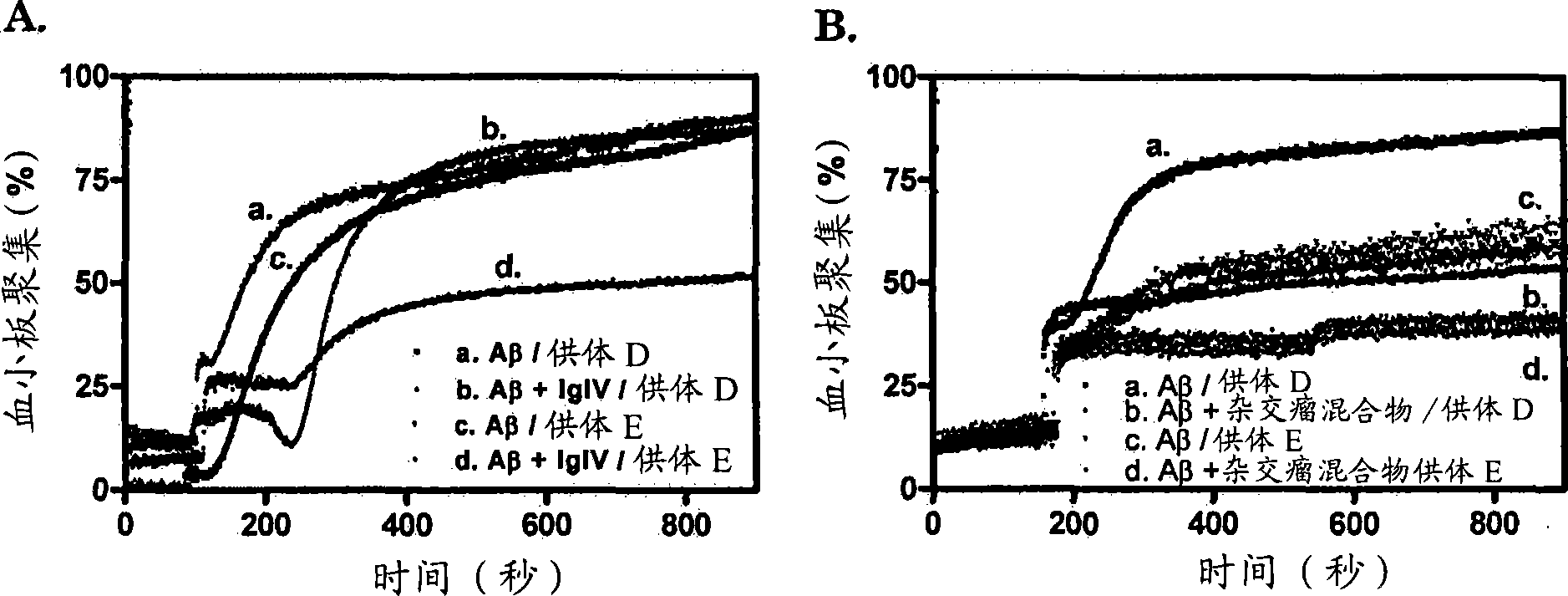
The present invention provides a method for selecting from a collection of IgIV molecules, at least one IgIV molecule comprising an affinity region that is capable of interacting with a misfolded protein and / or with an epitope of a cross-ss structure and / or with an epitope of a protein comprising a cross-beta structure, said method comprising contacting a collection of IgIV molecules with a misfolded protein and / or with a cross-ss structure and / or with a protein comprising a cross-beta structure and collecting at least one IgIV molecule comprising an affinity region interacting with said misfolded protein and / or epitope.
Owner:CROSSBETA BIOSCIENCES BV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com