Refolding of Recombinant Proteins
a recombinant protein and refolding technology, applied in the field of refolding of recombinant proteins, can solve the problems of cellular machinery, cellular machinery, and often expensive media for animal cell culture,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Solubilizing and Refolding of Recombinant human VEGF Expressed in Escherichia coli
Methods
[0116]Plasmid for VEGF165 expression—The plasmid pVEGF171 is designed for the expression of human VEGF165 (see, e.g., Leung et al., (1989) Science, 246: 1306-1309) in the E. coli periplasm. Transcription of the VEGF coding sequence is placed under tight control of the alkaline phosphatase (AP) promoter (see, e.g., Kikuchi et al., (1981) Nucleic Acids Research, 9:5671-8), while sequences required for translation initiation are provided by the trp Shine-Dalgarno region (see, e.g., Yanofsky et al., (1981) Nucleic Acids Research, 9:6647-68). The VEGF coding sequence is fused downstream of the bacterial heat-stable enterotoxin II (STII) signal sequence (see, e.g., Lee et al., (1983) Infect. Immun. 42:264-8; and, Picken et al., (1983) Infect. Immun. 42:269-75) for subsequent secretion into the E. coli periplasm. Codon modifications in the STII signal sequence provide for an adjusted translation level...
example ii
ingle Step Solubilization and Refolding of Recombinant human VEGF Expressed in Escherichia Coli
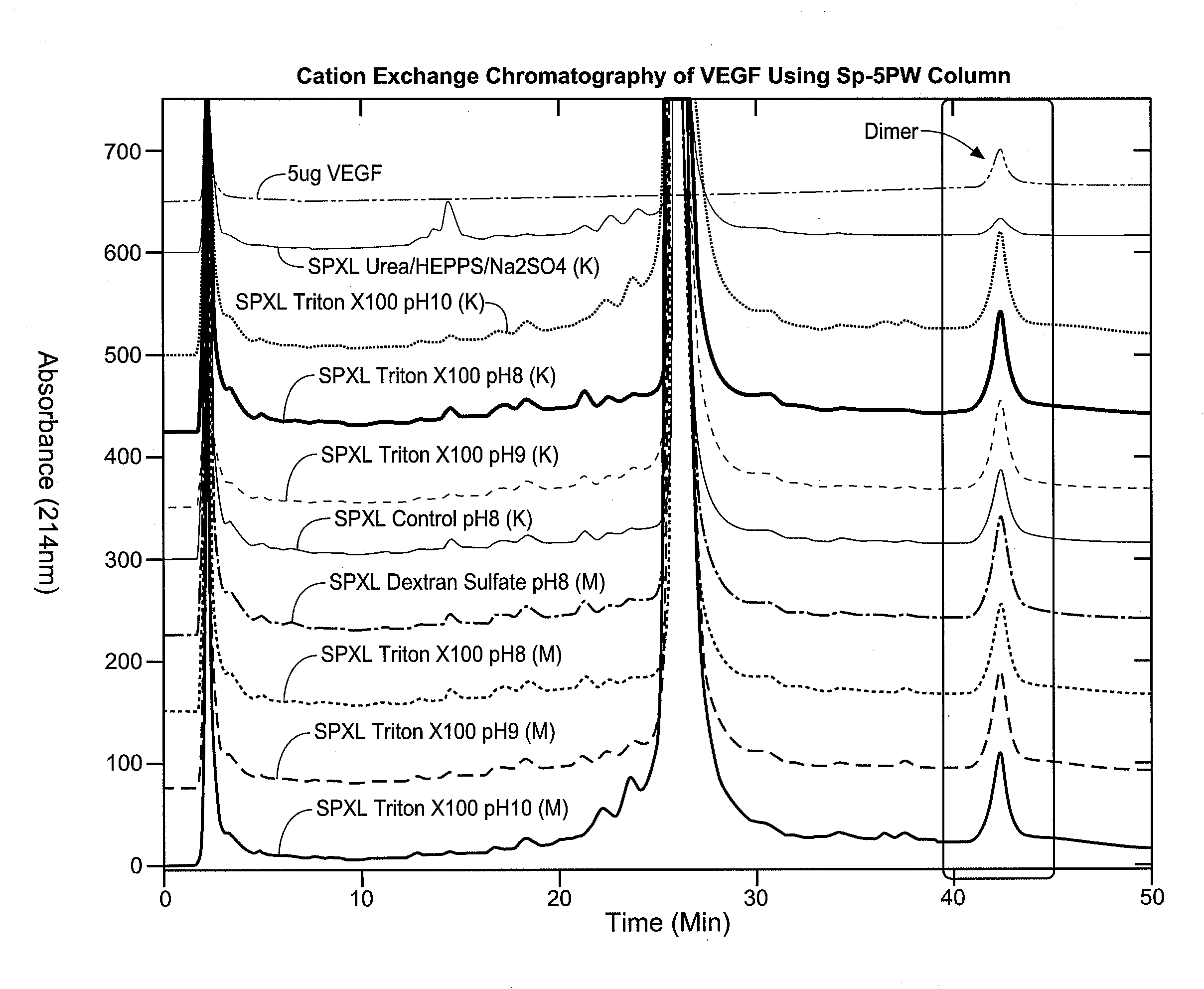
[0120]Solubilization and Refolding—The pellet is suspended in 10-39 liter volumes of refolding buffer (in this case termed “combo buffered solution”) for every kg of cell pellet, where the combo buffered solution contains 1 M Urea, 15 mM cysteine, 0.5 or 2 mM DTT, 100 mM arginine, 10 mM Tris or CHES, 5 mM EDTA, pH 9.5-10.5, final concentration. See FIG. 6 for the effect of urea and arginine addition in the refolding buffered solution. FIG. 6 shows the results of a 1-step pellet refold (combo buffered solution) as described in this example at pH 9.5 for 15 hours at room temperature. The denaturant concentrations are varied as follows: (1) 1 M urea and 100 mM arginine; (2) 1 M urea (and 0 mM arginine); (3) 2 M urea (and 0 mM arginine), while all the other buffer components (e.g., Tris or CHES, DTT, etc.) remain in the same concentration. The VEGF titer extracted from these is equivalent as ...
example iii
Non-Pellet Refolding of Recombinant Protein
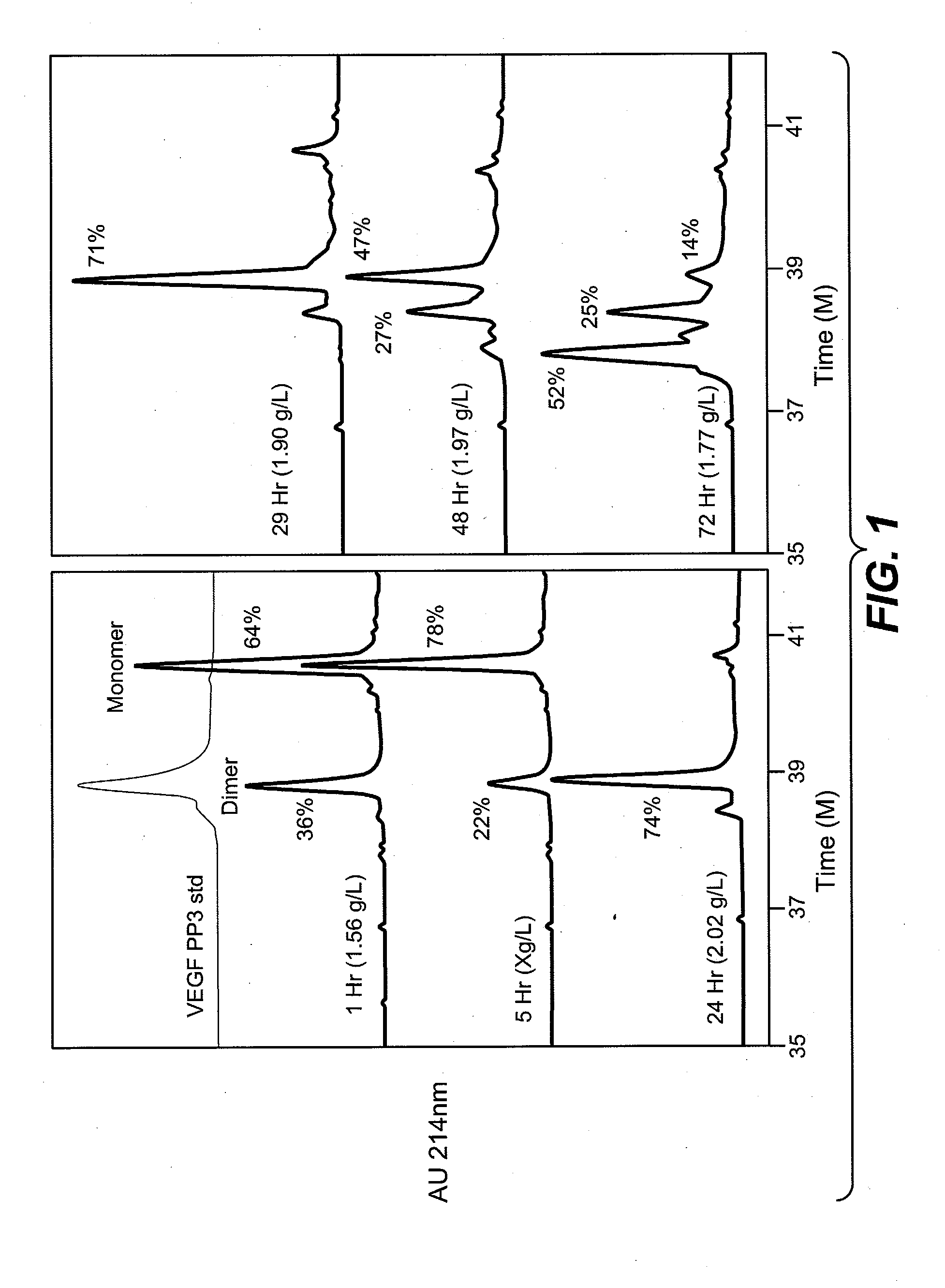
[0122]Escherichia coli whole cell broth producing recombinant protein is homogenized in a model 15 M laboratory homogenizer Gaulin 15M (small scale) or M3 (large scale) (Gaulin Corporation, Everett, Mass.) and diluted 1:4 (v / v) in refolding buffer per volume of homogenate and sparging with air at a mass transfer coefficient kLa=0.004 to 0.01 min−1, (e.g., For 2.5L vessel with marine type impeller, the air sparging rate would be 0.3 to 3 cc / min / L, or 0.3-1 cc / min / L, or 1 cc / min / L, or 3 cc / min / L while mixing is 200-400 rpm). The refolding buffer contains 1 M Urea, 15 mM cysteine, 2 mM DTT, 100 mM arginine, 10 mM Tris or CHES, 5 mM EDTA, pH 9-10, final concentration. Refold incubation is conducted at room temperature for 3-24 hours. Optionally, VEGF can be stabilized in the refold buffer by adding nitrogen in place of air after 3 hours refold incubation. The folding is monitored by cation exchange HPLC, rpHPLC chromatography, and / or Heparin HP...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com