Affinity regions
A technology of affinity region and protein, applied in the fields of biochemistry, molecular biology, structural biology and medicine, can solve problems such as harmful side effects, achieve the effects of preventing harmful side effects, improving therapeutic effect, and improving performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
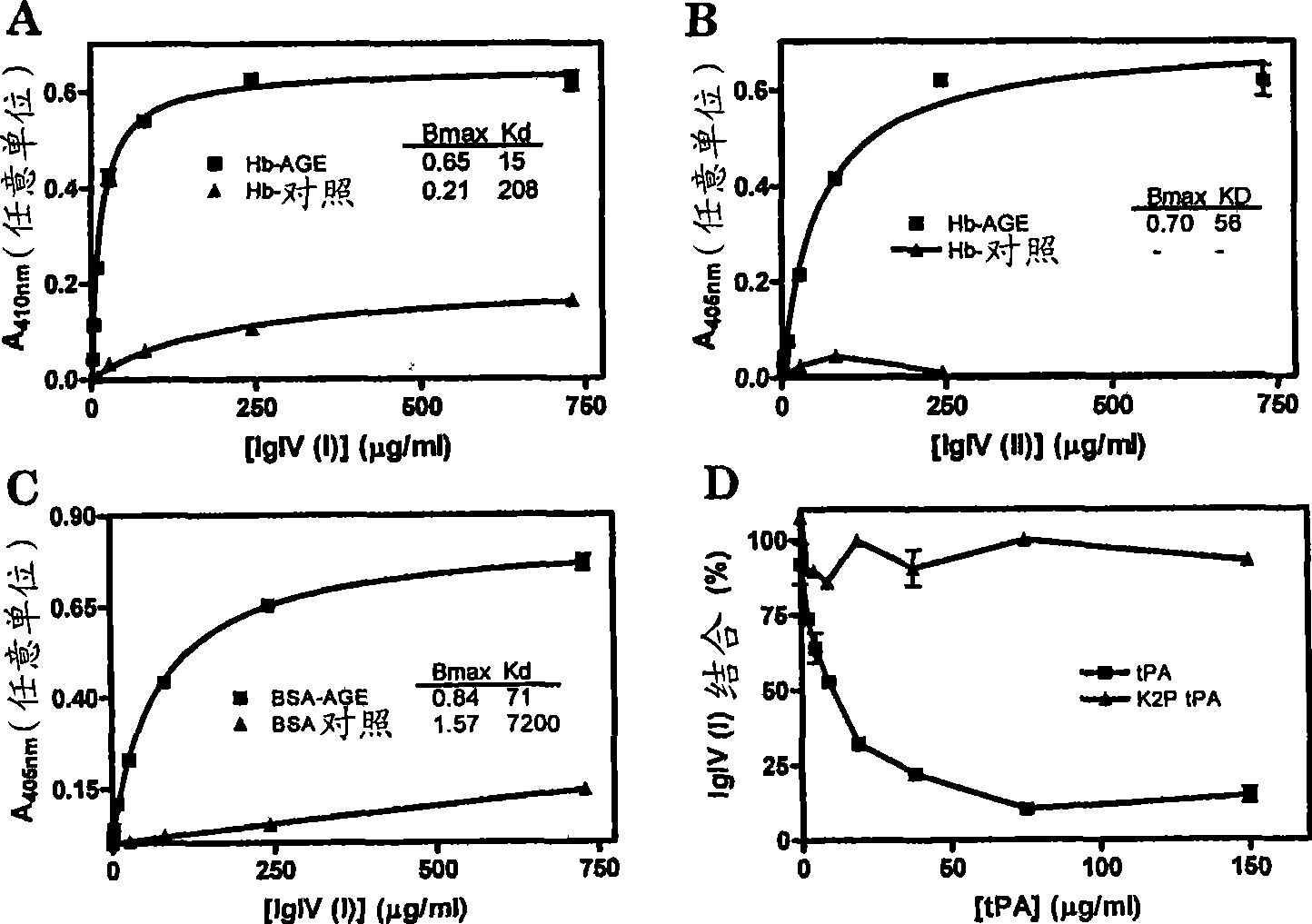
[0201] Example 1: IgIV (human immunoglobulin IgG antibody) binds misfolded proteins comprising a cross-beta structural conformation
[0202] Non-enzymatic modification of proteins by carbohydrates (hydrocarbons), a process called glycation, induces protein misfolding with formation of amyloid cross-beta structures ((Bouma et al., 2003). IgIV on immobilized The combination of glycated protein Hb-AGE and BSA-AGE and non-glycated Hb and BSA was constructed by ELISA device ( figure 1 A-C). IgIV binding was detected using alkaline phosphatase-labeled anti-human IgG or IgM antibodies. Both IgIV(I) and IgIV(II) bind with high affinity to glycated proteins containing cross-beta structures, whereas they bind weakly to immobilized native albumin and native hemoglobin ( figure 1 A-C). IgIV(I) has a higher affinity for immobilized proteins than IgIV(II). IgIV(I) has a higher affinity for Hb-AGE than for BSA-AGE. Depending on the albumin or hemoglobin preparation, slightly different...
Embodiment 2
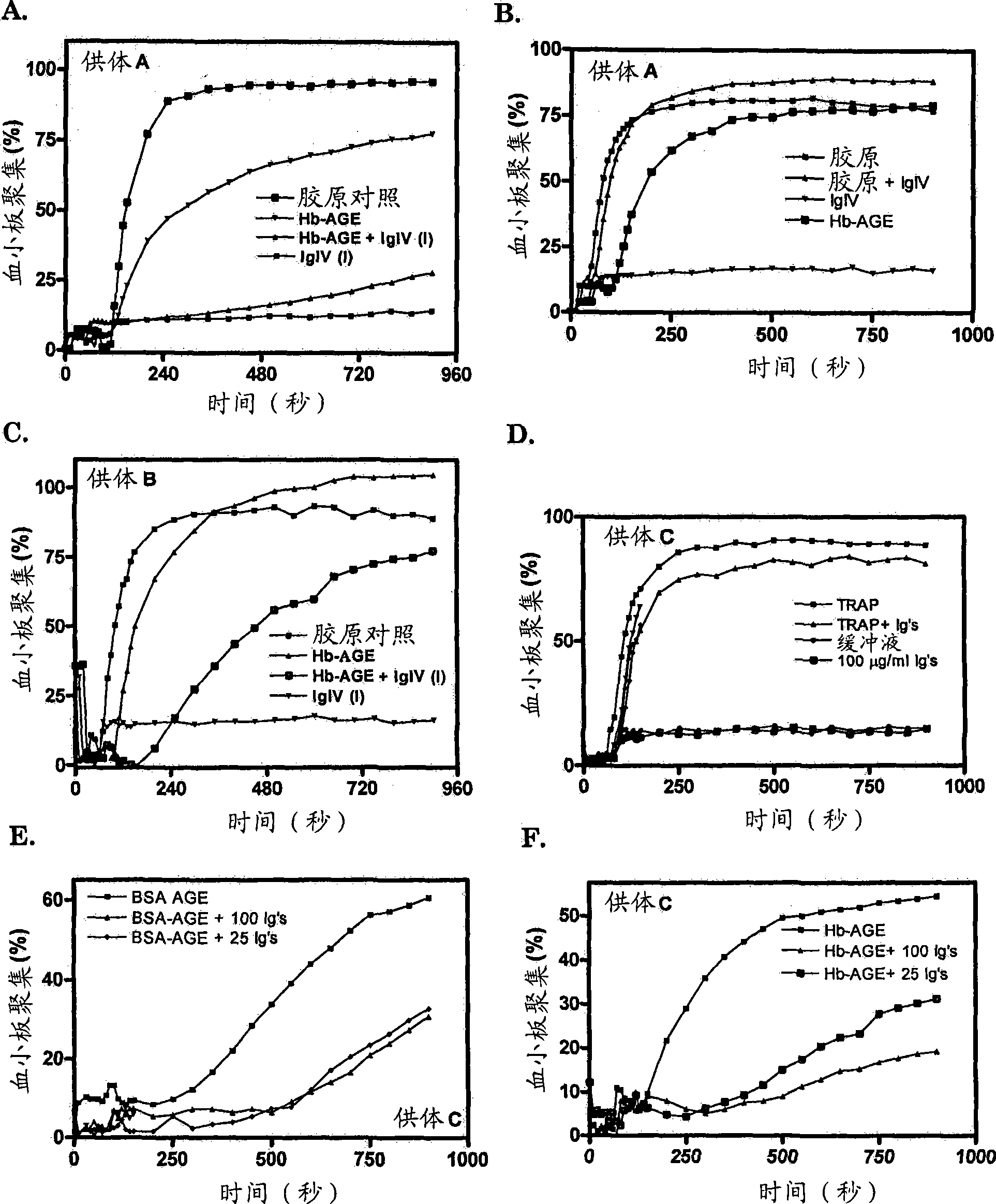
[0206] Example 2: Platelet aggregation is induced by amyloid misfolded protein and inhibited by human IgIV and mouse monoclonal antibodies
[0207] Platelets isolated from freshly citrated blood of apparently healthy human volunteers were prone to aggregate when exposed to misfolded glycosylated proteins, as from three different individuals with Hb-AGE (for "A", "B", "C") platelet confirmed ( figure 2 ). When the misfolded protein Hb-AGE or BSA-AGE was pre-incubated with IgIV(I) ( figure 2 A, C) or incubation with a mixture of 5 monoclonal antibodies (2E2B3D12, 7H2H2, 7H1C6A7, 7H9B9, 8F2G7H7) with affinity for misfolded proteins containing cross-beta conformation ( figure 2 E, F), platelet aggregation was inhibited. Induction of platelet aggregation by collagen or TRAP was poorly affected by IgIV(I) or mixed monoclonal antibodies ( figure 2 B, D), showing that these monoclonal antibodies are specifically known to be mediated by proteins containing cross-[beta] structur...
Embodiment 3
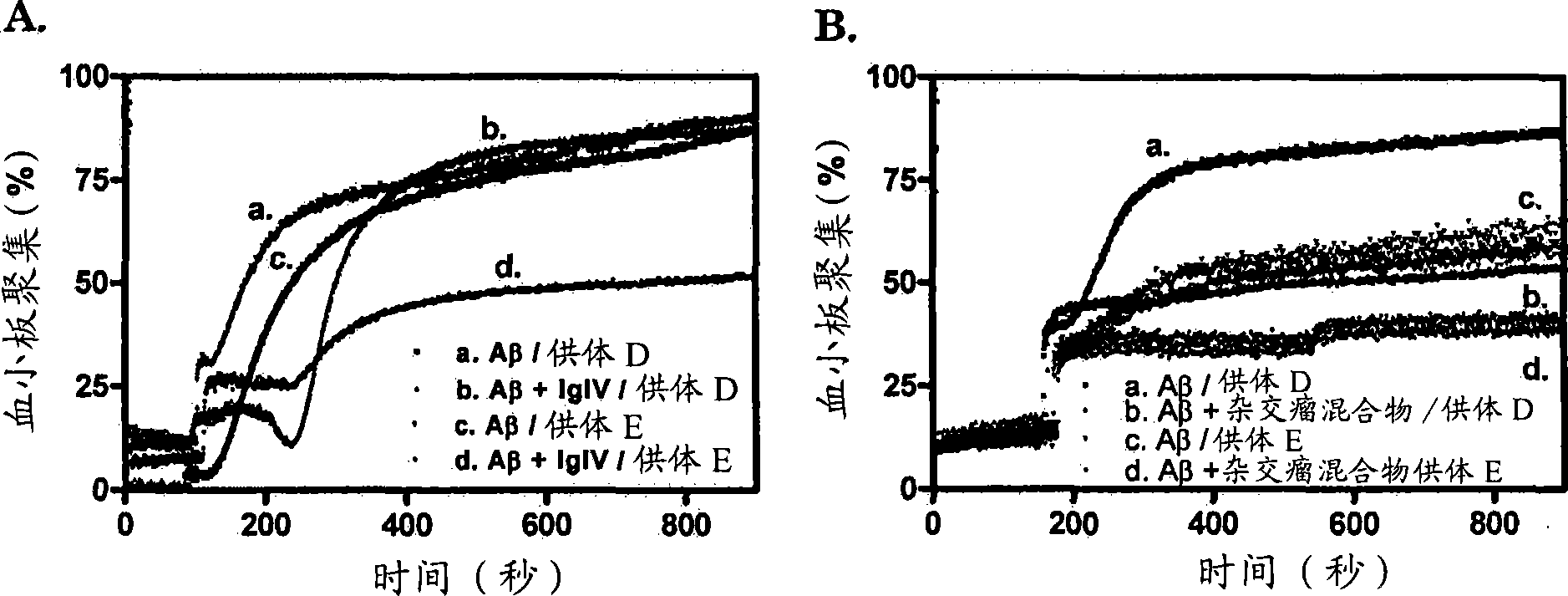
[0210] Example 3: Enhancement of binding of IgIV and tPA to misfolded proteins by Thioflavin T and Thioflavin S
[0211] Two amyloid-specific dyes, Thioflavin T and Thioflavin S, somewhat inhibit the IgIV-glycosylated protein interaction at relatively low dye concentrations, while at relatively high dye concentrations, both dyes appear to promote IgIV Binding to immobilized misfolded proteins ( Figure 4 B, C). This is explained by the fact that the binding of amyloid-specific dyes to misfolded proteins facilitates the subsequent binding of proteins that have binding affinity to misfolded proteins. Thioflavin T and Thioflavin S binding stabilize surrounding molecules or portions of molecules with a cross-beta structural conformation in a relatively fixed state representing the binding site for IgIV. At low dye concentrations, these forces were too weak to trigger immobilization into the IgIV binding sites of the more uniform exposed cross-[beta] structures. Now, dye binding...
PUM
| Property | Measurement | Unit |
|---|---|---|
| fluorescence | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com