Preparation method of insulin glargine and analogue thereof
An insulin analog, insulin glargine technology, applied in the field of preparation of insulin glargine and its analogs, can solve the problems of low yield, high cost, low separation yield and the like, and achieves high yield, simple method and application wide range of effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Embodiment 1: Utilize Escherichia coli expression system to construct genetically engineered bacteria
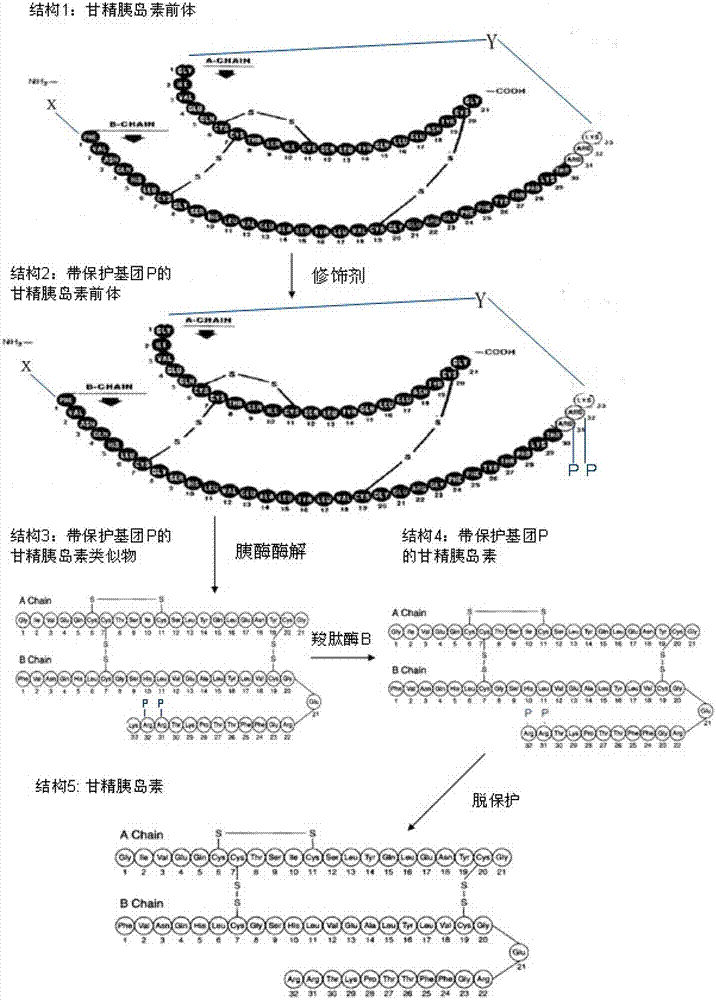
[0052] The preferred codons of Escherichia coli were selected, and the gene fragment expressing the insulin glargine precursor was obtained by fusion PCR technology, and the translated amino acid sequence was F V N Q H L CG S H L V E A L Y L V C G E R G F F YT P K T R R K E A E D L Q V G Q V E LG G G P G A G S L Q P L A L E G S L QR K G I V E Q C C T S I C S LY Q L EN Y C G, in order to improve the expression amount and renaturation efficiency , a piece of protein is fused at the N-terminus to connect with the connecting peptide GSK. The fusion sequence selected in this embodiment is a piece of staphylococcal protein A (SPA), M A D N K F N K E Q Q N A F Y E I LH L P N L N E E Q R N G F I Q S L K DD P S Q S A N L L A E A K K L N D AQ A P K A D N K. After introducing the NdeI EcoR I restriction site and connecting it into the expression vector pET 17b, the genetically en...
Embodiment 2
[0053] Example 2: Construction of genetically engineered bacteria using Pichia pastoris expression system
[0054] The preferred codons of Pichia pastoris were selected, and the gene fragment expressing the insulin glargine precursor was obtained by fusion PCR technology, so that the translated amino acid sequence was F V N Q H L CG S H L V E A L Y L V C G E R G F F YT P K T R R K G I V E Q C C T S I C S LY Q L E N Y C G, in order to improve the cleavage efficiency of the signal peptide and ensure the N-terminal Integrity, add the connection fragment K R E E A E A E A E PK at the N-terminus, introduce the Xho I EcoR I restriction site and connect it into the vector pPIC 9, and then clone it into the expression vector pPIC 9k with Sal I / Sac I, after conventional genetic engineering The genetically engineered bacteria expressing the insulin glargine precursor were obtained. After fermentation, the fermentation broth was taken for Tricine SDS-PAGE analysis. The results are shown i...
Embodiment 3
[0055] Embodiment 3: crude pure expression product
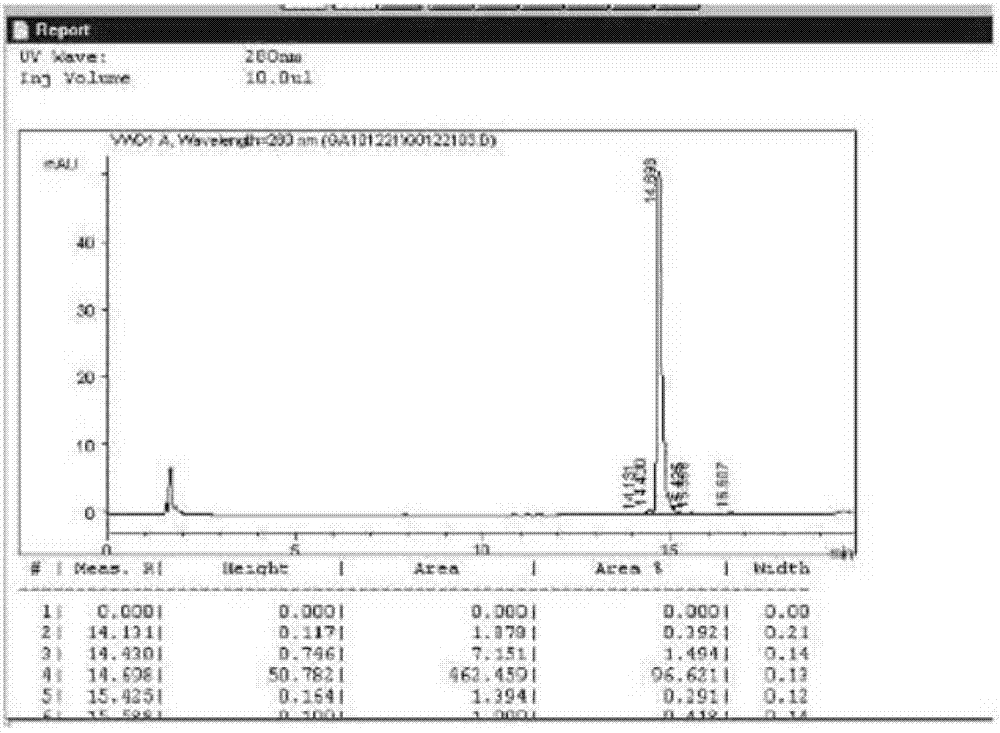
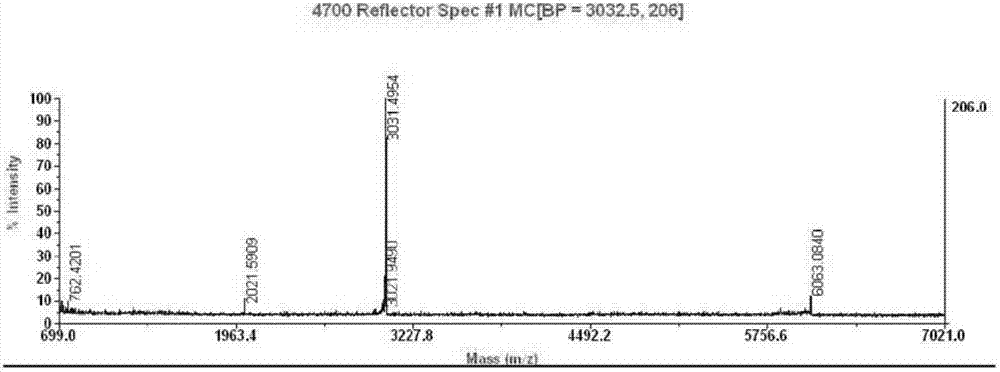
[0056] Escherichia coli expression product in Example 1, use 50mM PB+2mM EDTA, pH7.5 to break the bacteria; use 50mM Tris-HCl+2mM EDTA+0.5%Triton X-100, pH8.0; 50mMTris-HCl+2mM EDTA+1M Wash the bag sequentially with Urea, pH8.0; denature with 50mM Tris-HCl+2mM EDTA+8M Urea, pH9.0; sulfonate with 50mM Tris-HCl+2mM EDTA+8M Urea+0.3M Na2SO3, pH9.0; 2mM EDTA+1M Urea+1mM β-Me, pH 9.5 after renaturation, put on Q column, pH 8.0 salt concentration gradient elution to obtain crude precursor protein, SDS-PAGE analysis, the results are shown in the appendix Figure 4 , the results show that the precursor protein with higher purity can be obtained after renaturation crude purification, the molecular weight is about 17.8KD, and the main impurity is the dimer formed in the renaturation process.
[0057] In Example 2, the yeast expression product was diluted and the pH was adjusted to 4.0, and the CM column was eluted with a pH and salt ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com