A method for preparing insulin glargine crystals
An insulin glargine and crystallization technology is applied in the field of preparing insulin glargine crystals, which can solve the problems of short centrifugation time and freeze-drying time, influence the quality of insulin products, and bring risks to the crystallized products, and achieve simple operation, uniform size and shape, easy-to-control effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The insulin glargine crystal liquid is prepared, and the contents of each component in the crystal liquid are: insulin glargine: 2.53 g / L, phenol: 0.1%, zinc chloride: 0.05%, citric acid: 0.5%.
[0036] Weigh 10.11g of insulin glargine and dissolve it in 2L of water to make insulin glargine solution, add 3.99g of phenol, 2.02g of zinc chloride, and 20.11g of citric acid to the crystallization solution, add water to make up to 4L, and make crystallization liquid. Stir the crystallization solution at room temperature at a low speed, adjust the pH value to 5.0 with sodium hydroxide, stir for 4 hours, let it stand at room temperature, and then take the supernatant for testing. The content of insulin glargine in the supernatant is 0.04mg / ml .
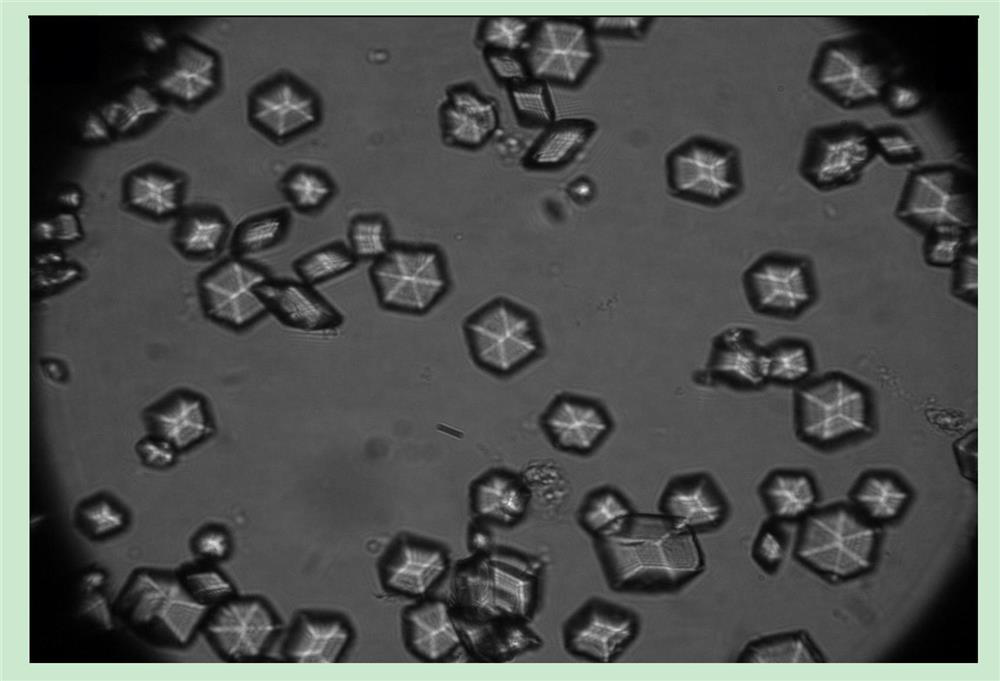
[0037] Take the crystal suspension for microscopic examination, and magnify 1000 times to see obvious hexahedral crystals with high transparency ( figure 1 ).
Embodiment 2
[0039] Insulin glargine crystal liquid is prepared, the content of each component in the crystal liquid is: insulin glargine: 2.07g / L, phenol: 0.01%, zinc chloride: 0.007%, citric acid: 0.05%.
[0040] Weigh 2.07g of insulin glargine and dissolve it in 500ml of water to make insulin glargine solution, add 2ml of 5% phenol, 1.75ml of 4% zinc chloride, and 0.5g of citric acid to the crystallization solution, add water to make up to 1L, Made into a crystallization solution. Stir the crystallization solution at room temperature at a low speed, adjust the pH to 6.8 with sodium hydroxide, stir for 6 hours, let it stand at room temperature, and then remove the supernatant for testing. The content of insulin glargine in the supernatant is 0.01 mg / ml.
[0041] Take the crystal suspension for microscopic examination, magnification of 400 times shows obvious crystals, and the transparency is higher ( figure 2 ).
Embodiment 3
[0043] Insulin glargine crystal liquid was prepared, and the content of each component in the crystal liquid was: insulin glargine: 3.17g / L, phenol: 0.7%, zinc chloride: 1.5%, citric acid: 1.8%.
[0044] Weigh 6.34g of insulin glargine and dissolve it in 1L of water to make an insulin glargine solution. Add 14g of phenol, 30g of zinc chloride, and 36g of citric acid to the crystallization solution, add water to make the volume to 2L, and make a crystallization solution. Stir the crystallization solution at room temperature at a low speed, adjust the pH to 7.0 with sodium hydroxide, stir for 9 hours, let it stand at room temperature, and then remove the supernatant for detection. The content of insulin glargine in the supernatant is 0.04 mg / ml.
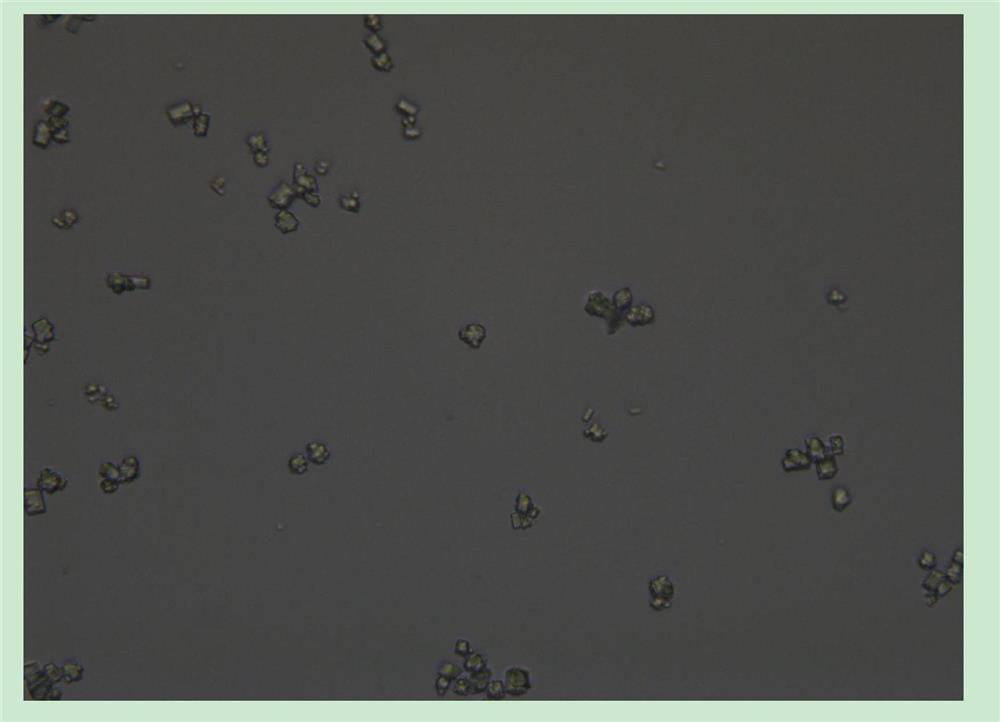
[0045] Take the crystal suspension for microscopic examination, and magnify 400 times to see obvious crystals, and the transparency is higher ( image 3 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com