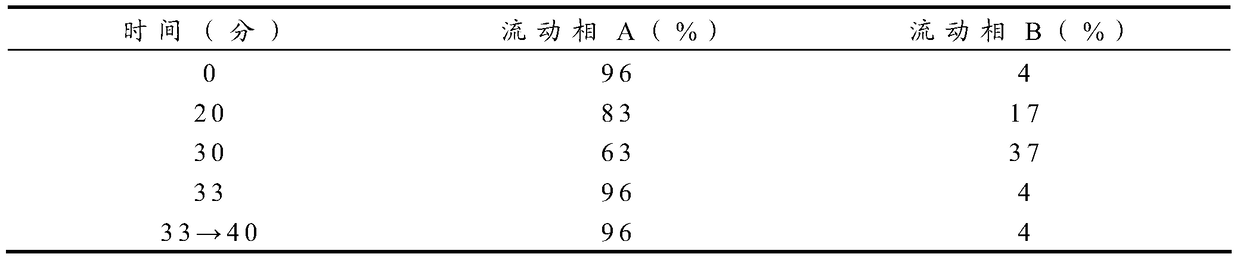
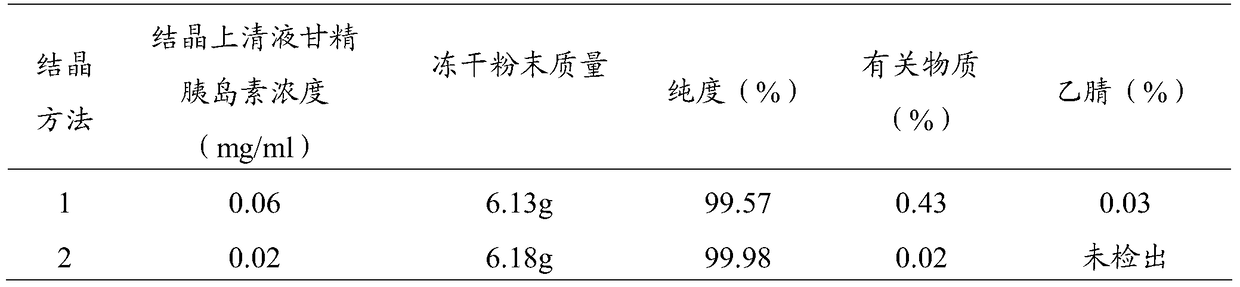
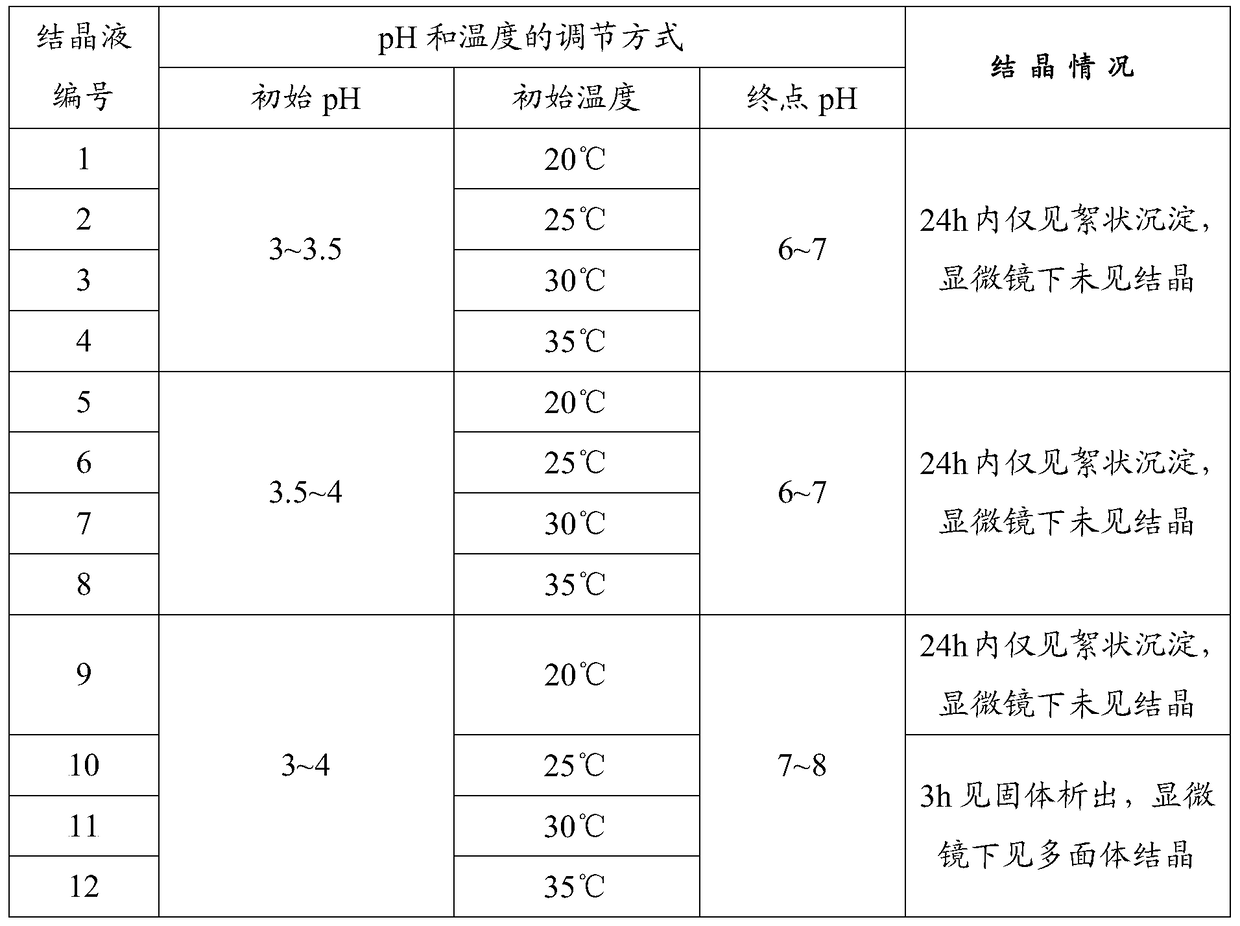
A kind of preparation method of insulin glargine and insulin glargine prepared thereof
An insulin glargine and crystallization technology, which is applied in the preparation methods of peptides, insulin, chemical instruments and methods, etc., can solve the problems of excessive residual solvent and increase the operation steps of crystal washing in the later stage, so as to achieve less impurities and solvent residues and improve production. The effect of simple rate and composition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Example 1 A kind of preparation method of recombinant insulin glargine crystal
[0058] 1) Prepare 1L of recombinant insulin glargine crystallization solution. The components and concentrations in the crystallization solution are: recombinant insulin glargine: 2g / L; acetic acid: 0.5mol / L; zinc chloride: 70mg / L; phenol: 3g / L L; specific operation process:
[0059] Measure 250ml of recombinant insulin glargine solution with a concentration of 8g / L; measure 28.6ml of acetic acid and dilute to 250ml with water; weigh 3g of phenol and dissolve it with as little water as possible; weigh 70mg of zinc chloride and dissolve it with as little water as possible dissolve;
[0060] Add pre-prepared acetic acid solution, phenol solution and zinc chloride solution to the recombinant insulin glargine solution, add water to make the volume to 1 L, and obtain the recombinant insulin glargine crystal solution.
[0061] 2) Stir the recombinant insulin glargine crystal solution obtained ...
Embodiment 2
[0065] Example 2 A kind of preparation method of recombinant insulin glargine
[0066] 1) Prepare 1L of recombinant insulin glargine crystallization solution. The components and concentrations in the crystallization solution are: recombinant insulin glargine: 4g / L; acetic acid: 0.8mol / L; zinc chloride: 100mg / L; phenol: 5g / L L; specific operation process:
[0067] Measure 500ml of recombinant insulin glargine solution with a concentration of 8g / L; measure 45.7ml of acetic acid and dilute to 300ml with water; weigh 5g of phenol and dissolve it with as little water as possible; weigh 100mg of zinc chloride and dissolve it with as little water as possible. dissolve;
[0068] Add recombinant insulin glargine solution, acetic acid solution and zinc chloride solution to the pre-prepared phenol solution, add water to make the volume to 1 L, and obtain the recombinant insulin glargine crystal solution.
[0069] 2) Stir the recombinant insulin glargine crystal solution obtained in s...
Embodiment 3
[0073] Example 3 A kind of preparation method of recombinant insulin glargine crystal
[0074] 1) Prepare 1L of recombinant insulin glargine crystallization solution. The components and concentrations in the crystallization solution are: recombinant insulin glargine: 1g / L; acetic acid: 0.4mol / L; zinc oxide: 40mg / L; phenol: 2g / L ; Specific operation process:
[0075] Measure 125ml of recombinant insulin glargine solution with a concentration of 8g / L; measure 22.8ml of acetic acid and dilute to 150ml with water; weigh 2g of phenol and dissolve it in as little water as possible; weigh 40mg of zinc oxide and use as little as 0.1mol / L is dissolved in hydrochloric acid;
[0076] Add the recombinant insulin glargine solution, acetic acid solution and phenol solution to the pre-prepared zinc salt solution, add water to make the volume up to 1L, and obtain the recombinant insulin glargine crystal solution.
[0077] 2) Stir the recombinant insulin glargine crystal solution obtained...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com