Extraction method of insulin glargine precursor protein
A technology for insulin glargine and precursor protein, which is applied in the field of extraction of insulin glargine precursor protein, can solve the problems that the recovery rate of precursor protein is not significantly improved, and the protein content is not increased, so as to achieve the content of insulin glargine precursor protein Improve and compensate for the loss of the target protein and improve production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Microbial strain: Pichia pastoris GS115 (commercially available)
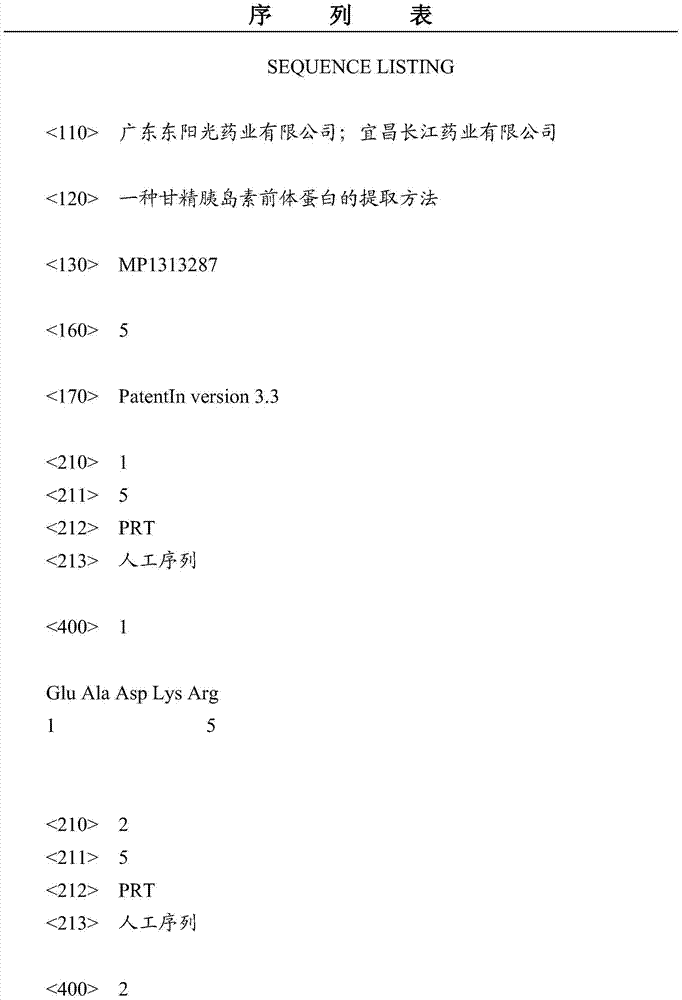
[0029] C peptide sequence: amino acid sequence shown in SEQ ID NO:1
[0030] Fermentation broth: obtained by fermentation culture of Pichia pastoris GS115 introduced with the precursor gene of insulin glargine.
[0031] The pH value of the fermentation broth was measured to be 5.0, then the supernatant was centrifuged and the concentration of the precursor of insulin glargine was measured by HPLC as a standard value of 100 as a control, and then 16 parts of the fermentation broth of the same volume were taken, using 1.2mol / L hydrochloric acid and glacial acetic acid , 10% concentrated sulfuric acid and 95% phosphoric acid were adjusted to pH 1, 2, 3, 4, and then centrifuged to take the supernatant for comparison. The results are shown in Table 1.
[0032] Table 1 HPLC detection results of insulin glargine precursors adjusted by different acids
[0033] pH
[0034] It can be seen from Table 1 that by adding aci...
Embodiment 2
[0036] Microbial strain: Pichia pastoris GS115 (commercially available)
[0037] C peptide sequence: amino acid sequence shown in SEQ ID NO:1
[0038] Fermentation broth: same as Example 1
[0039] The pH of the fermentation broth was measured to be 5.0, then the supernatant was centrifuged and the concentration of the precursor of insulin glargine was measured by HPLC as a standard value of 100 as a control, and then 10 parts of the fermentation broth of the same volume were taken, using 1.2mol / L hydrochloric acid and 95% Phosphoric acid was adjusted to pH 1.2, 1.5, 1.8, 2.1, 2.4, and then centrifuged to take the supernatant for comparison. The results are shown in Table 2.
[0040] Table 2 HPLC detection results of insulin glargine precursors adjusted by different acids
[0041] pH
[0042] It can be seen from Table 2 that adding acid treatment according to the method of the present invention, when the pH value is 1.2, 1.5, 1.8, 2.1, 2.4, all acids significantly increase the conce...
Embodiment 3
[0044] Microbial strain: Saccharomyces cerevisiae MT663 (commercially available)
[0045] C peptide sequence: amino acid sequence shown in SEQ ID NO:1
[0046] Fermentation broth: obtained by fermentation culture of Saccharomyces cerevisiae MT663 introduced with the precursor gene of insulin glargine.
[0047] The pH of the fermentation broth was measured to be 5.2, then the supernatant was centrifuged and the concentration of the precursor of insulin glargine was measured by HPLC as a standard value of 100 as a control, and then 10 parts of the fermentation broth of the same volume were taken, using 1.2mol / L hydrochloric acid and 95% Phosphoric acid was adjusted to pH 1.2, 1.5, 1.8, 2.1, 2.4, and then centrifuged to take the supernatant for comparison. The results are shown in Table 3.
[0048] Table 3 HPLC detection results of insulin glargine precursors adjusted by different acids
[0049] pH
[0050] It can be seen from Table 3 that adding acid treatment according to the method ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com