A kind of preparation method of insulin glargine crystal
A technology of insulin glargine and crystal, which is applied in the field of insulin crystallization, can solve the problems of analysis, many interfering impurities and low purity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
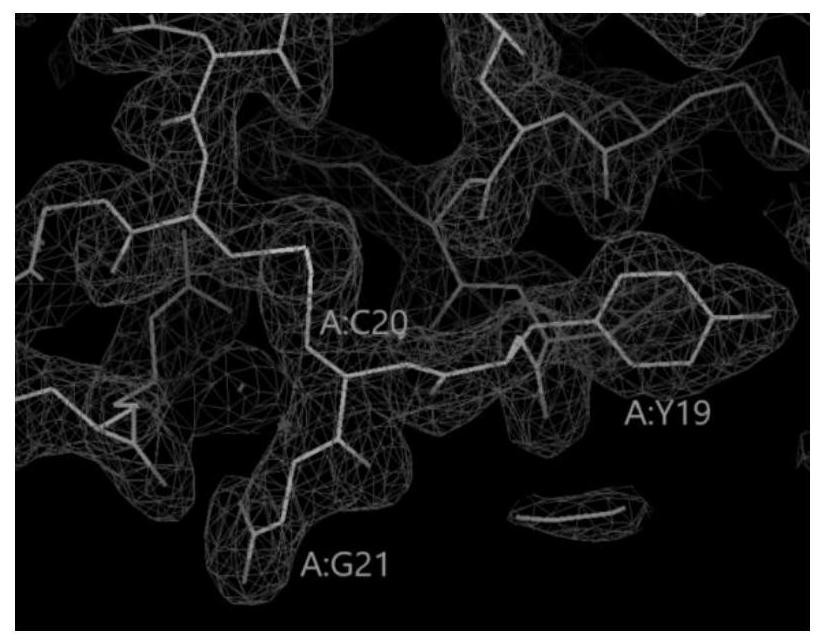
[0032] This example provides a method for preparing insulin glargine crystals.
[0033] 1. Preparation of stock solution
[0034] 1M citric acid-1M Tris buffer solution: Weigh 19.2g of anhydrous citric acid and 12.1g of Tris, add 100ml of water to dissolve, adjust the pH with NaOH solution, and obtain a buffer solution with a pH value of 8.0-11.0.
[0035] 1M ammonium acetate solution: weigh 0.77g of ammonium acetate, add 10ml of water to dissolve it.
[0036] 4MNaOH: Weigh 1.16g of sodium hydroxide, add 5ml of water to dissolve.
[0037] 1M phenol solution: weigh 94mg of phenol, add 1ml of water to dissolve, and mix well.
[0038] 0.5M zinc acetate solution: Weigh 219mg of zinc acetate, add 2ml of water to dissolve, and mix well.
[0039] 0.01M hydrochloric acid solution: Measure 0.9ml hydrochloric acid, dilute it with water to 1000ml, and shake well.
[0040] 2. Preparation of insulin glargine solution
[0041] Weigh 20mg of insulin glargine, add 5ml of 0.01M hydrochlor...
Embodiment 2
[0060] This example provides a method for preparing insulin glargine crystals. Compared with Example 1, the only difference is that the composition of the crystallization solution is: Tris 0.4M, citric acid 0.4M, ammonium acetate 25mM; the pH of the crystallization solution is 8.5.
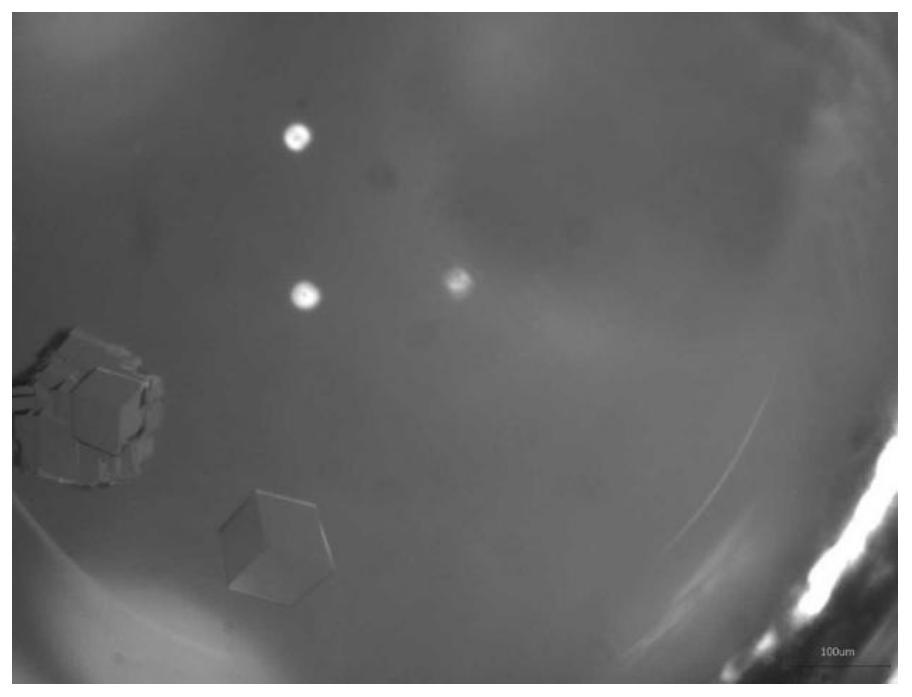
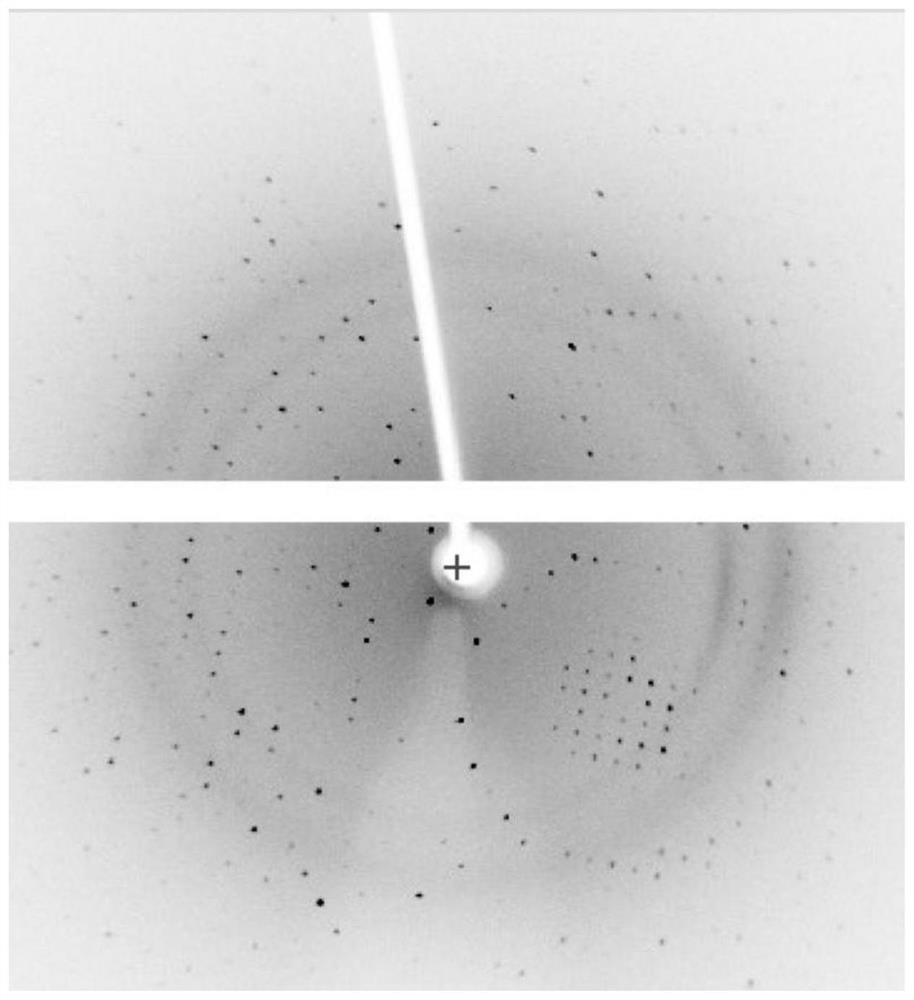
[0061] In this embodiment, insulin glargine crystals in the form of single crystals can be obtained.
Embodiment 3
[0063] This example provides a method for preparing insulin glargine crystals. Compared with Example 1, the only difference is that the composition of the crystallization solution is: Tris 0.7M, citric acid 0.7M, ammonium acetate 30mM; the pH of the crystallization solution is 9.0.
[0064] In this embodiment, insulin glargine crystals in the form of single crystals can be obtained.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com