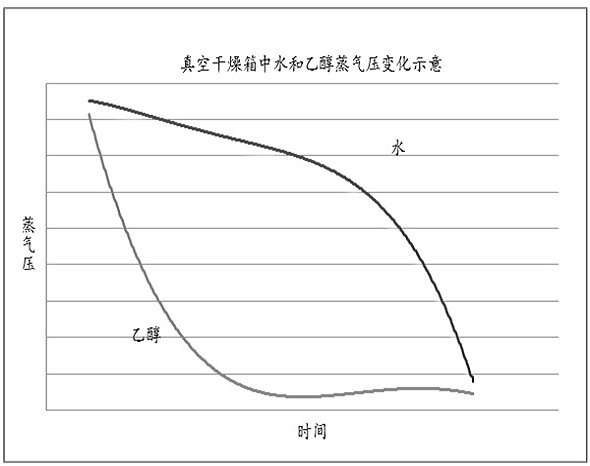
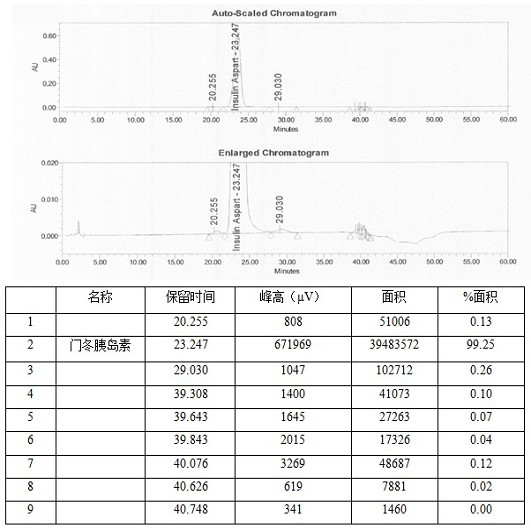
Method for efficiently removing residual organic solvent in insulin aspart
A technology of residual organic solvent and insulin aspart, applied in the field of biomedicine, can solve the problems of long drying time, unfavorable industrial amplification, increase production cost, etc., and achieve the effect of stable product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] Embodiment 1 (the mass ratio of the addition of water and medicine is 1:2.5)
[0072] Step A: Put 4.7 Kg of insulin aspart wet solid (117ppb ethanol solvent residue) into a tray, crush the large solid, control the thickness of the wet solid to 8.8 mm, put it in a vacuum drying oven, close the door, and open Vacuum pump, open the vacuum valve to make the vacuum pressure be -0.884 bar ~ -0.886 bar. Turn on the heater, control the temperature at 22°C during the process, and dry in vacuum for 24 h to remove most of the residual organic solvents that are easy to remove.
[0073] Step B: keep the pressure and moisturize: close the vacuum valve, slowly open the empty valve, when the vacuum pressure drops to zero, open the door of the vacuum drying oven, crush the large solid, turn over the material, then put the tray in the vacuum drying oven and add 1.9 Kg pure water, turn on the vacuum pump to vacuumize, make the vacuum pressure be -0.867 bar ~ -0.884 bar, then turn off the...
Embodiment 2
[0098] Example 2 (the mass ratio of the amount of water added to the drug is 1:1)
[0099] Step A: Put 5.8Kg of insulin aspart wet solid (residual amount of ethanol solvent is 117ppb) into a tray, crush the large solid, control the thickness of the wet solid to 10.1 mm, put it in a vacuum drying oven, close the door, and open Vacuum pump, open the vacuum valve to make the vacuum pressure be -0.939 bar ~ -0.991 bar. Turn on the heater, control the temperature at 22°C during the process, and dry in vacuum for 24 hours to remove most of the residual organic solvents that are easy to remove.
[0100] Step B: Keeping the pressure and moisturizing: close the vacuum valve, slowly open the empty valve, when the vacuum pressure drops to zero, open the door of the vacuum drying oven, crush the large solids, turn over the material, put the tray in the vacuum drying oven after turning over and dry Add 5.8Kg of pure water, turn on the vacuum pump to vacuumize, make the vacuum pressure be ...
Embodiment 3
[0109] Example 3 (moisture is added twice, the mass ratio of the total amount of water added to the drug is 1:2)
[0110] Step A: Put 5.18Kg of insulin aspart wet solid (residual amount of ethanol solvent is 117ppb) into a tray, crush the large solid, control the thickness of the wet solid to 9.5 mm, put it in a vacuum drying oven, close the door, and open Vacuum pump, open the vacuum valve so that the vacuum pressure is -0.831 bar ~ -0.844 bar. Turn on the heater, control the temperature at 22°C during the process, and dry in vacuum for 24 hours to remove most of the residual organic solvents that are easy to remove.
[0111] Step B: Keeping the pressure and moisturizing: close the vacuum valve, slowly open the empty valve, when the vacuum pressure drops to zero, open the door of the vacuum drying oven, crush the large solids, turn over the material, put the tray in the vacuum drying oven after turning over and dry Add 1.3Kg of pure water, turn on the vacuum pump for vacuumi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com