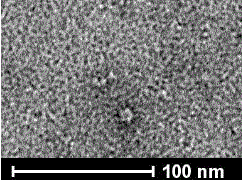
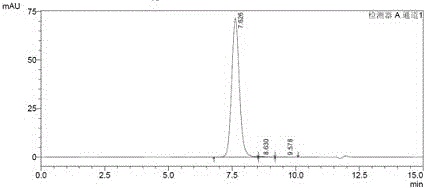
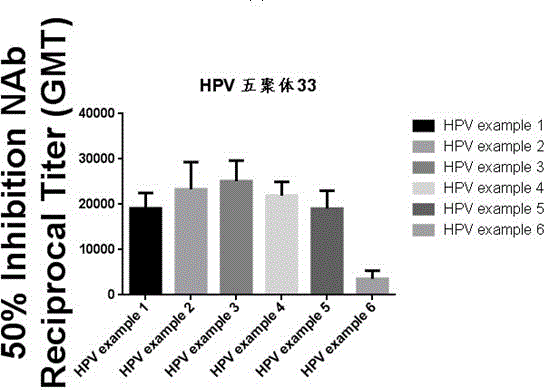
Recombinant human papilloma virus type 33 L1 protein and its purpose
A protein and combination technology, applied in the direction of viruses, viral peptides, antiviral agents, etc., can solve the problems of difficult target proteins, many types of hybrid proteins, loss of natural conformation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment l
[0072] Embodiment 1: have the construction of the engineering bacterium of HPV33 L1 sequence 2
[0073] 1、 The full-length HPV33 L1 gene was synthesized by GENEWIZ Biotechnology Co., Ltd. (GENEWIZ). Nucleotide sequence SEQ ID NO: 1, which is derived from GeneBank, the sequence number is GenBank: M14119.1.
[0074] 2. A template for PCR reaction containing the gene fragment of SEQ ID NO:1. With the forward primer sequence: 5'- CGCGGA TCCGGA GAAAAGGAAGACCCTTAGGT -3'; a restriction endonuclease AccIII site was introduced at the 5' end of the H4 structure, and the sequence of the AccIII site was TCCGGA. The downstream contains the XhoI endonuclease site, the reverse primer sequence: 5'-GCTCTCCTCGAG TTA TTTAGGTTTT GCTTTAAGAC C -3', the 5' end introduces the restriction endonuclease XhoI site, and the XhoI site sequence is CTCGAG. HPV33B was amplified by PCR reaction.
[0075] 3. A template containing the gene fragment of SEQ ID NO: 1 for PCR reaction. To contain the introduce...
Embodiment 2
[0080] Embodiment 2: Construction of engineering bacteria with HPV33 L1 sequence 4
[0081] 1. The target gene fragment of the full-length HPV33 L1 gene was purchased from the clinical cell sample waste containing wild-type HPV33 virus from the Gynecology Clinic of Beijing Anzhen Hospital. Nucleotide The sequence is SEQ ID NO: 3 (GenBank: FN870689.1).
[0082] 2. A template containing the gene fragment of SEQ ID NO: 3 for PCR reaction. With the forward primer sequence: 5'- CGCGGATCCGGA GAAAAGGAAGACCCTTAGGT -3'; a restriction endonuclease AccIII site was introduced at the 5' end of the H4 structure, and the sequence of the AccIII site was TCCGGA. The downstream contains the XhoI endonuclease site, the reverse primer sequence: 5'-GCTCTCCTCGAG TTA TGTGCGGGTG GATGTGGGGG C -3', its 5' end introduces the restriction endonuclease XhoI site, and the XhoI site sequence is CTCGAG. After PCR reaction, HPV33B was amplified.
[0083] 3. A template containing the gene fragment of SE...
Embodiment 3
[0088] Embodiment 3: Construction of engineering bacteria with HPV33 L1 sequence 6
[0089] 1、 The full-length HPV33 L1 gene was synthesized by GENEWIZ Biotechnology Co., Ltd. (GENEWIZ). Nucleotide sequence SEQ ID NO: 5, which is derived from GeneBank, the sequence number is GenBank: FN870694.1.
[0090] 2. A template containing the gene fragment of SEQ ID NO: 3 for PCR reaction. Forward primer sequence: 5'- CGCGGATCCGGA GAAAAGGAAGACCCCTTAGGT-3'; a restriction endonuclease AccIII site was introduced at the 5' end of the H4 structure, and the sequence of the AccIII site was TCCGGA. The downstream contains the XhoI endonuclease site, the reverse primer sequence: 5'-GCTCTCCTCGAG TTA TGTGCGGGTG GATGTGGGGG C -3', its 5' end introduces the restriction endonuclease XhoI site, and the XhoI site sequence is CTCGAG. PCR reaction, amplified to obtain HPV33B.
[0091]3. A template containing the gene fragment of SEQ ID NO: 3 for PCR reaction. To contain the introduced restriction en...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com