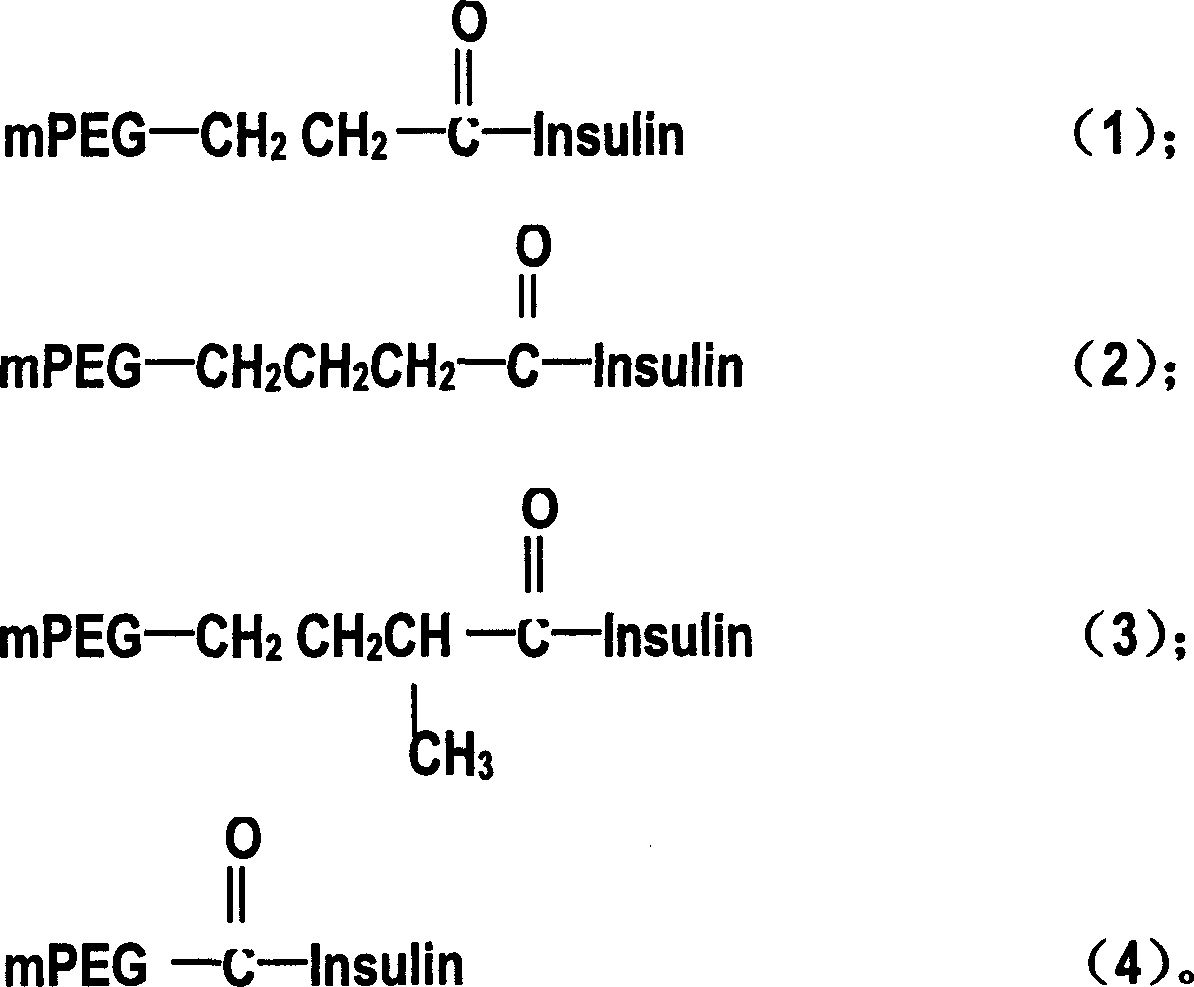Oral insulin compound medicine preparation and its preparing method
A compound preparation and insulin technology, applied in the field of medicine, can solve problems such as troublesome use and drug effect destruction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
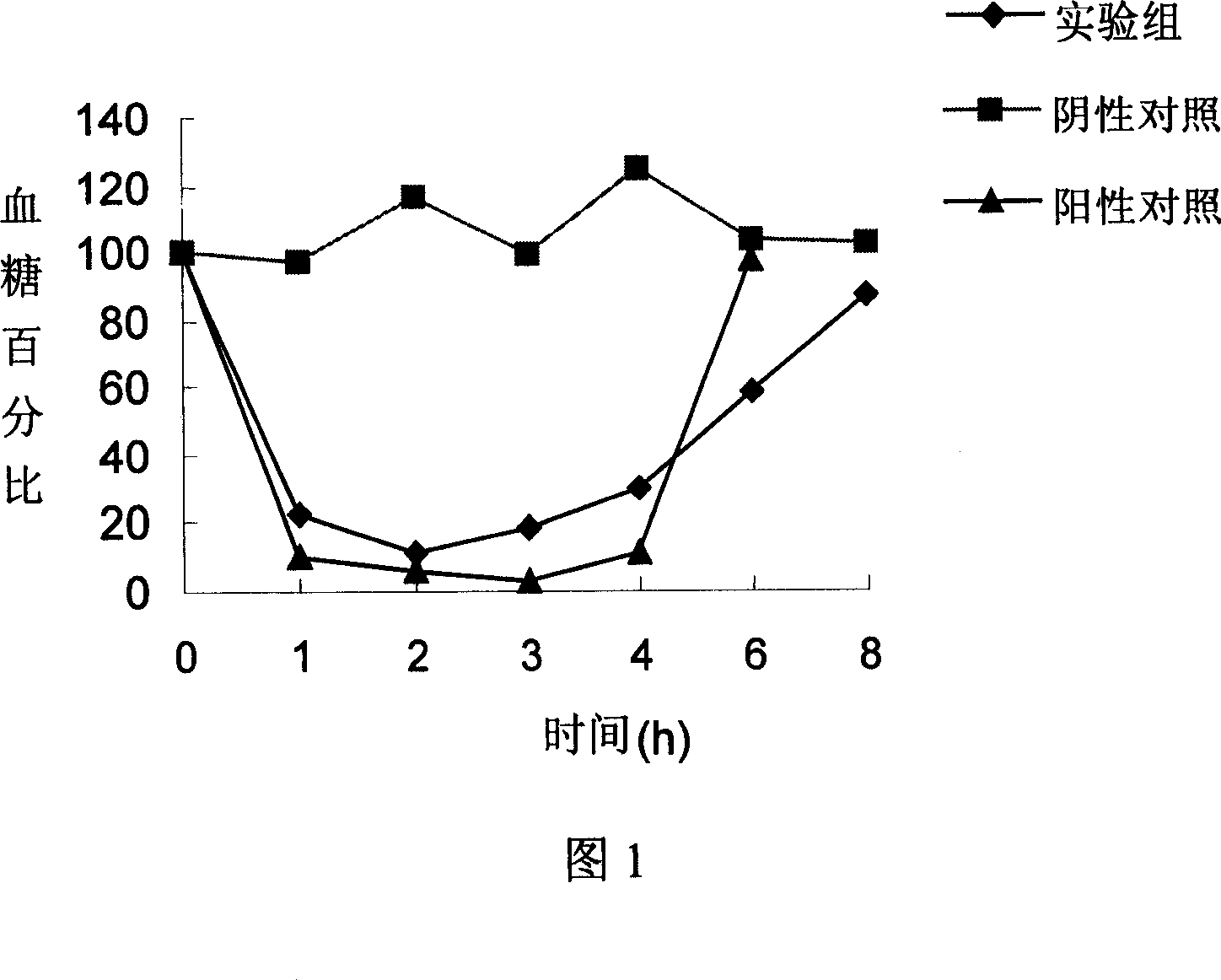
[0093] 1. Dissolve 100 mg of recombinant human insulin lyophilized powder in 5 ml of 0.01 mol / L HCl solution, add 100 ml of 1% PEG 3350 (polyethylene glycol, or one or more of molecular weight 2000-8000, the following same);
[0094] 2. Add 5ml 125mmol / L CaCl in sequence 2 (or other calcium-containing compounds such as calcium nitrate, etc.) and 1ml of 165mmol / L Na 3 C 6 h 5 o 7 , the pH is adjusted to 6-9 to make A solution;
[0095] 3. Dissolve 1000mg of casein in the buffer solution of phosphorus-containing compounds (such as phosphoric acid, phosphate solution) to make B solution with a casein concentration of 3mg / ml;
[0096] 4. Add solution B to solution A, stir for 24-48 hours, and the reaction temperature is 4-5°C;
[0097] 5. Vacuum freeze-drying to prepare the oral insulin compound preparation of the present invention.
example 2
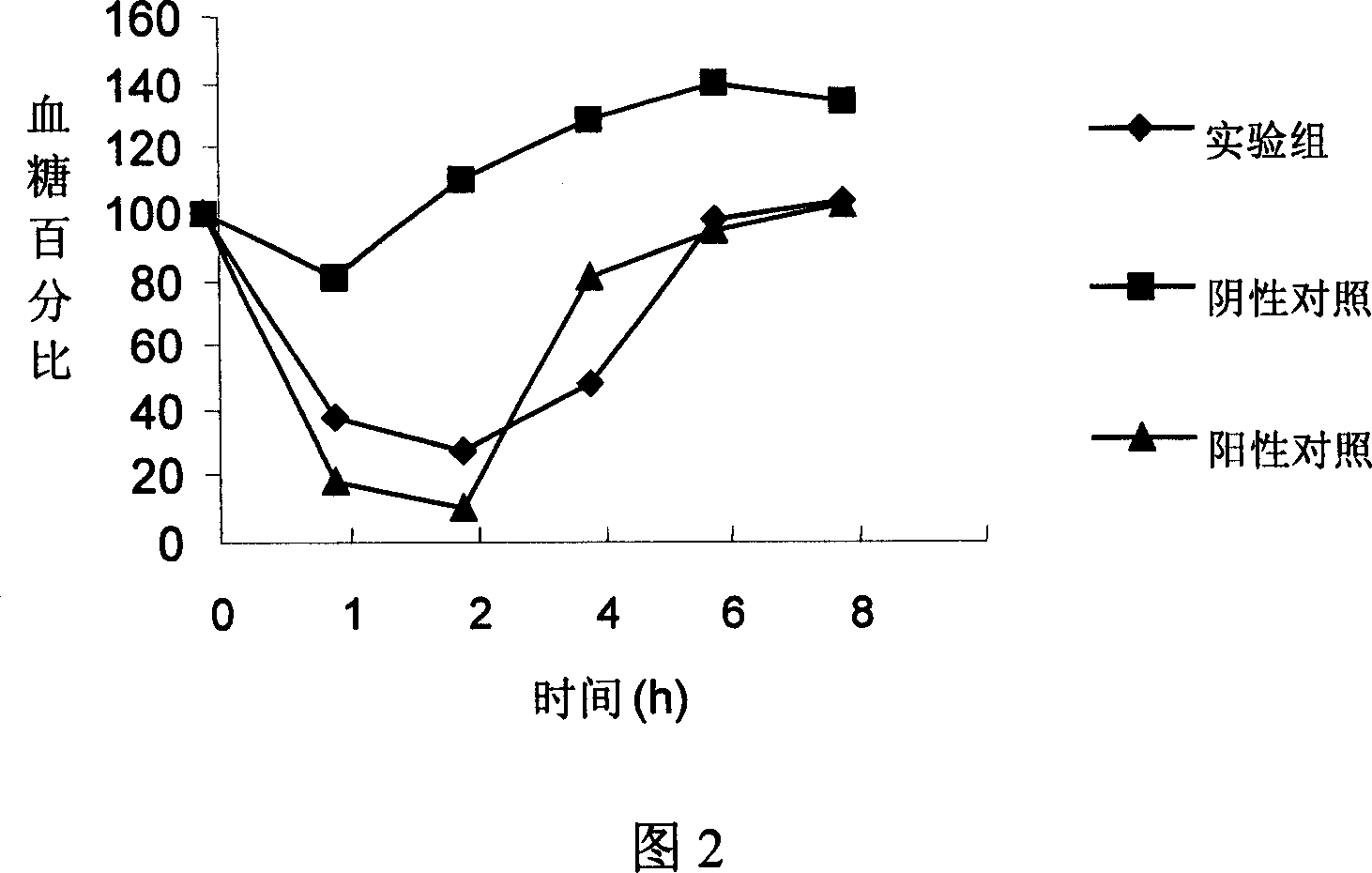
[0099] 1. Dissolve 100mg of recombinant human insulin lyophilized powder in 5ml of 0.01mol / L HCl solution, add 100ml of 1% PEG 3350;
[0100] 2. Add 5ml 125mmol / L CaCl in sequence 2 and 1ml of 165mmol / L Na 3 C 6 h 5 o 7 , adjust the pH to be 6-9;
[0101] 3. Mix 5ml 125mmol / L Na 2 HPO 4 Add to the above solution, stir for 24-48 hours; vacuum freeze-dry to make freeze-dried product;
[0102] 4. Dissolve 1000mg casein in phosphate buffer solution, the concentration is 3mg / ml;
[0103] 5. Add the freeze-dried product to casein phosphate buffer, stir and mix well, and the reaction temperature is 4-5°C;
[0104] 6. Vacuum freeze-drying to prepare the oral insulin compound preparation of the present invention.
[0105] Add 600-800 mg of the absorption promoter sodium deoxycholate to the lyophilized product of the oral insulin compound preparation described in Example 1-2, and fill 200 mg of the disintegrating agent microcrystalline cellulose into enteric-coated capsules to pr...
example 3
[0113] 1. Dissolve 20umol of insulin in 10ml of 60%DMF / 40%H at room temperature 2 O (pH9.6);
[0114] 2. Dissolve 28umol of mPEG-SPA in 1.5ml of DMF, and add it to the insulin solution for reaction, add 1mol / L NaOH to maintain the reaction pH, react at room temperature for about 30 minutes, then add 25ml H 2 O ends the reaction;
[0115] 3. Use 0.01% NH in the reaction mixture 4 HCO 3 Dialysis, lyophilization after dialysis to obtain monomethoxypolyethylene glycol derivative-insulin composite preparation;
[0116] 4. Dissolve the monomethoxypolyethylene glycol derivative-insulin compound preparation in 5ml of water, add 100ml of 1% PEG 3350, mix well, then add 5ml of 125mmol / L CaCl in turn 2 and 1ml of 165mmol / L Na 3 C 6 h 5 o 7 , adjust the pH value to 6-9 to make A solution;
[0117] 5. Dissolve 1000mg of casein in phosphate buffer to make B solution with a casein concentration of 3mg / ml;
[0118] 6. Add solution B into solution A, stir for 24-48 hours, and the rea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com