Recombinant insulin and insulin analogue precursor purification method
A technology for insulin analogs and purification methods, which is applied in the field of purification of recombinant insulin and insulin analog precursors, to achieve the effects of reducing process time and equipment investment costs, good process continuity, and simple operation steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
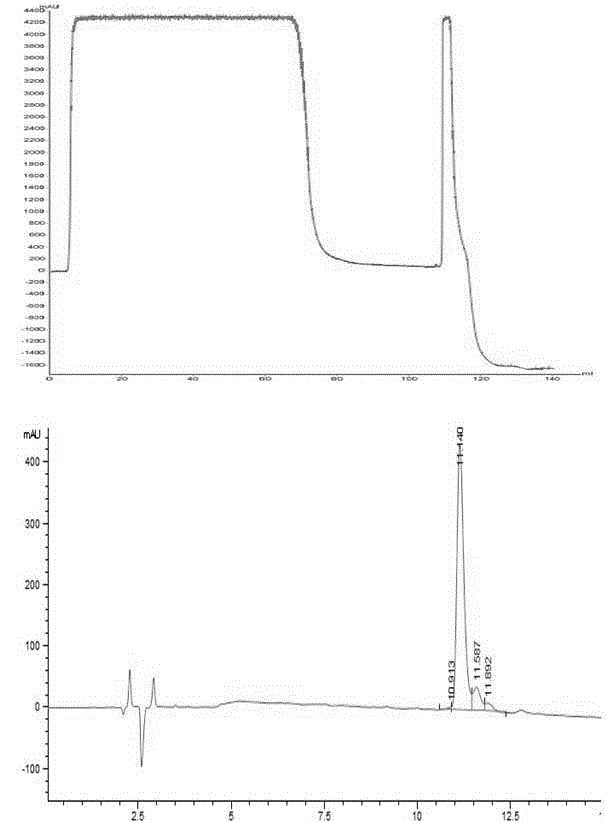
[0036] Example 1: Separation of recombinant human DesB using the filler CaptoS in this application and the reference filler SP-Sepharose6FF 30 insulin precursor
[0037] (1) Pretreatment of fermentation broth: the fermentation broth is derived from Pichia pastoris fermentation broth, adjusted to pH 2.0 with hydrochloric acid, centrifuged for 15 minutes, 8000g, 4-10°C, collected the centrifuged supernatant, and measured the conductivity value to 55mS / cm; The purity of the supernatant detected by HPLC was 38%, and the content was 3.5 mg / ml;
[0038] In order to test the influence of different conductivity on the purification results, the above-mentioned fermentation with a conductivity of 55mS / cm was adjusted to 3 parts of 40, 20 and 10mS / cm with water, and experiments were carried out respectively.
[0039] (2) Equipment / filler / buffer solution
[0040] Equipment: AKTAPureM1 chromatography equipment (GE Company, USA), full-wavelength ultraviolet detector, flow rate range 0.01-...
Embodiment 2
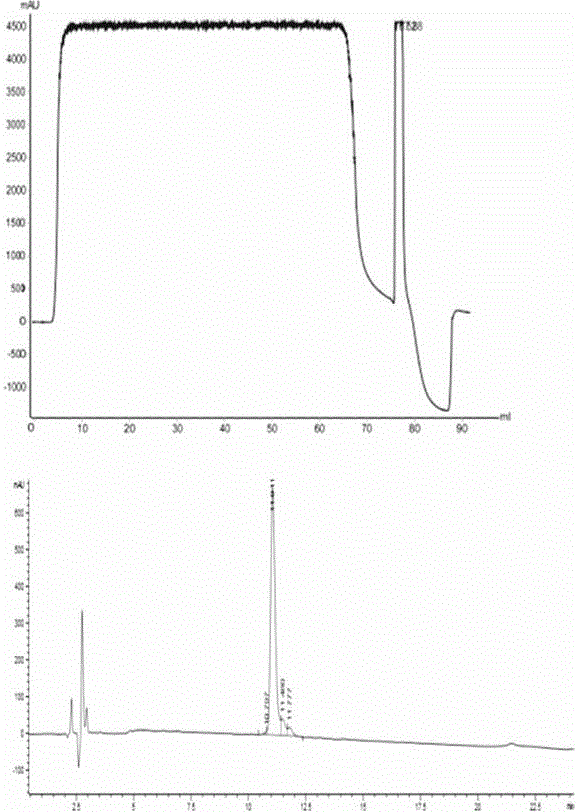
[0056] Example 2: Purification and separation of recombinant human DesB by CaptoMMC 30 insulin precursor
[0057] (1) Sample source: the same as in Example 1.
[0058] (2) the equipment / filler / buffer solution conditions used in this embodiment are as follows:
[0059] Equipment: AKTAPureM1 chromatography equipment (GE Company, USA), full-wavelength ultraviolet detector, flow rate range 0.01-20ml / min.
[0060] Chromatography column: XK16 / 20 column, detection wavelength: 280nm.
[0061] Chromatography packing material: CaptoMMC, column bed height: 10cm; column volume 20ml (self-filling);
[0062] Injection volume: 70-80ml, linear flow rate: 300cm / h, volumetric flow rate: 10ml / min.
[0063] Equilibrium buffer: 100mM glycine-hydrochloric acid buffer, pH2.0;
[0064] Elution buffer: 200 mM Tris-HCl buffer, pH 8.5.
[0065] (3) Operation steps: with embodiment 1.
[0066] (4) Experimental results
[0067] Table 2 CaptoMMC purification and isolation of recombinant human DesB ...
Embodiment 3
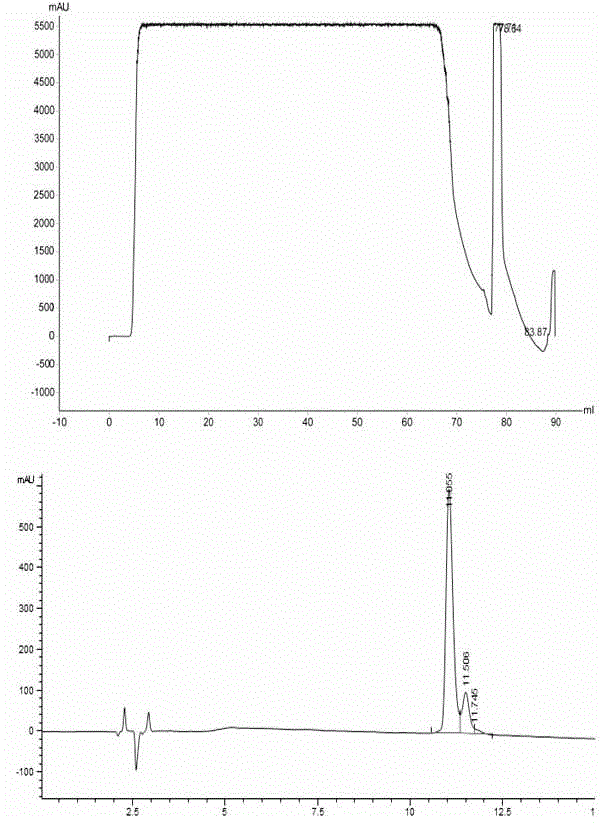
[0069] Example 3: Purification and separation of recombinant human DesB by UniPMMS 30 insulin precursor
[0070] (1) Sample source: the same as in Example 1.
[0071] (2) the equipment / filler / buffer solution conditions used in this embodiment are as follows:
[0072] Equipment: AKTAPureM1 chromatography equipment (GE Company, USA), full-wavelength ultraviolet detector, flow rate range 0.01-20ml / min.
[0073] Chromatography column: XK16 / 20 column, detection wavelength: 280nm.
[0074] Chromatography packing material: UniPMMS, column bed height: 10cm; column volume 20ml (self-filling);
[0075] Injection volume: 70-80ml, linear flow rate: 300cm / h, volumetric flow rate: 10ml / min;
[0076] Equilibrium buffer: 80mM citric acid-sodium citrate buffer, pH4.0;
[0077] Elution buffer: 100mM glycine-sodium hydroxide buffer, pH8.5;
[0078] (3) Operation steps: with embodiment 1.
[0079] (4) The experimental results are as follows:
[0080] Table 3 UniPMMS separation and purific...
PUM
| Property | Measurement | Unit |
|---|---|---|
| height | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com