Chromatographic purification method for acylated insulin
A technique for acylating insulin and chromatographic purification, which is applied in the field of preparation of recombinant insulin or insulin analogs, can solve the problems of low sample resolution, non-dense and uniform media, column collapse and column efficiency, etc., so as to avoid adverse reactions, The effect of saving purification cost and simple and feasible method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Preparation of Preliminary Example 1 Acylated Insulin
[0043] For the preparation method of the acylated insulin solution, refer to the method disclosed in Chinese Patent CN1171742A.
[0044] Biosynthetic human insulin (BHI) crystals (71.9 mg) were dissolved in 6.58 mL DMSO. The solution was stirred at room temperature until the crystals were completely dissolved (visual observation). A solution of active ester (N-succinimidyl palmitate) was prepared by adding 20 mg of active ester solid to 2 mL of DMSO and stirring vigorously until all active ester particles were completely dissolved (by visual inspection). At this point, 1,1,3,3-tetramethylguanidine (26.8 μl) was added to 5 mL of the BHI solution, followed by DMSO (94.4 mL) and the previously prepared active ester solution (400 μl). The reaction was carried out at room temperature (20 to 25°C) for about 60 minutes. A sample was taken after 15 minutes, diluted 20-fold with 1N acetic acid and analyzed by HPLC. B in...
Embodiment 2
[0054] Example 2 investigates the influence of phase A on the separation effect of samples under different pH elution conditions
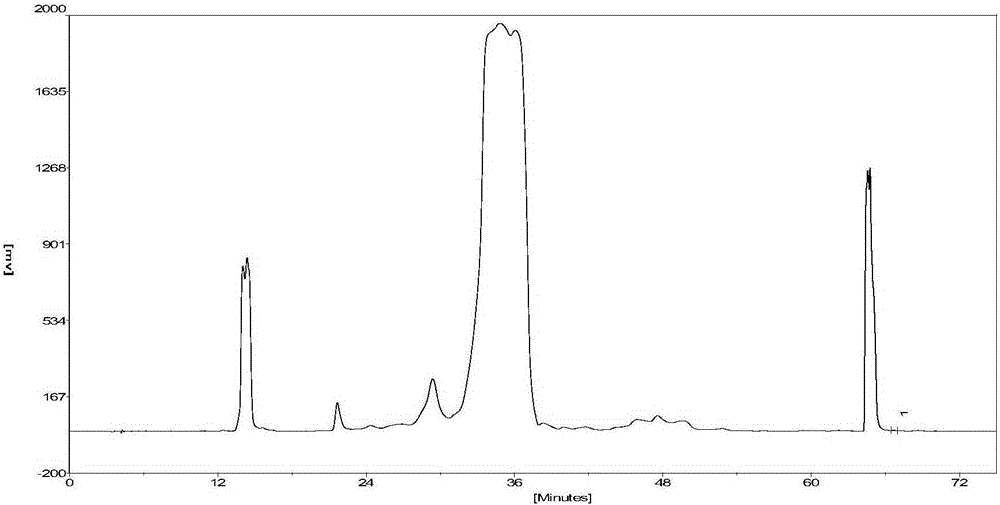
[0055] The crude sample is an acylated insulin solution prepared by one-step reaction of B30-removed recombinant human insulin and N-succinimidyl palmitate. The preparation method is the same as that in Preliminary Example 1. The crude sample has a purity of 56%; the chromatographic purification method Using high-grade silica gel carrier ( ) is carried out on a semi-preparative chromatographic column (50mm×250mm), and the filling pressure is 7-10MPa; the mobile phase A phase for elution: 20mmol / L disodium hydrogen phosphate-citric acid buffer, adjusted by hydrochloric acid or sodium hydroxide The pHs are 3.5 and 7.5 respectively; mobile phase B for elution: acetonitrile-water (mixed at a volume ratio of 9:1); keep the column temperature at 20-25°C; the elution flow rate is 82.5ml / min; the detection wavelength is 280nm .
[0056] The specific ste...
Embodiment 3
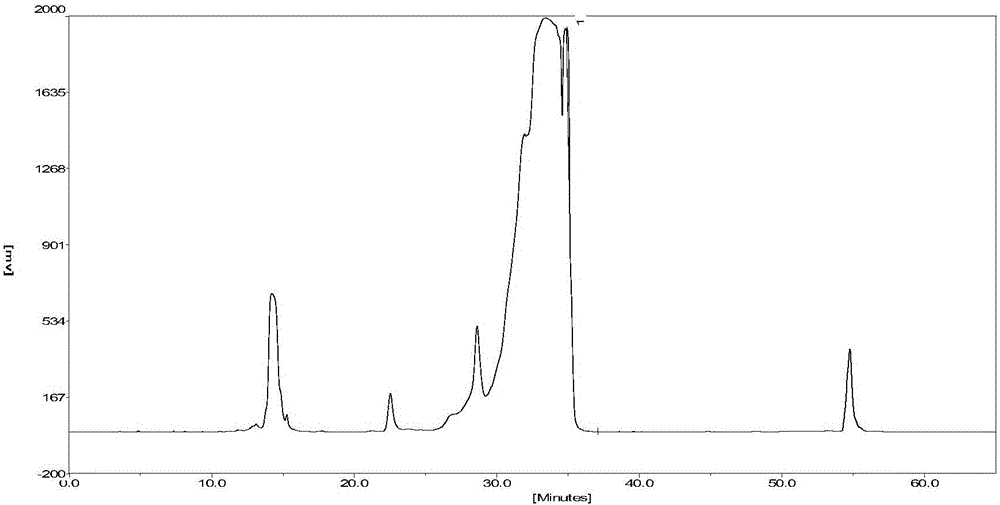
[0066]Example 3 Collect samples in sections to determine the purity and yield of samples after purification
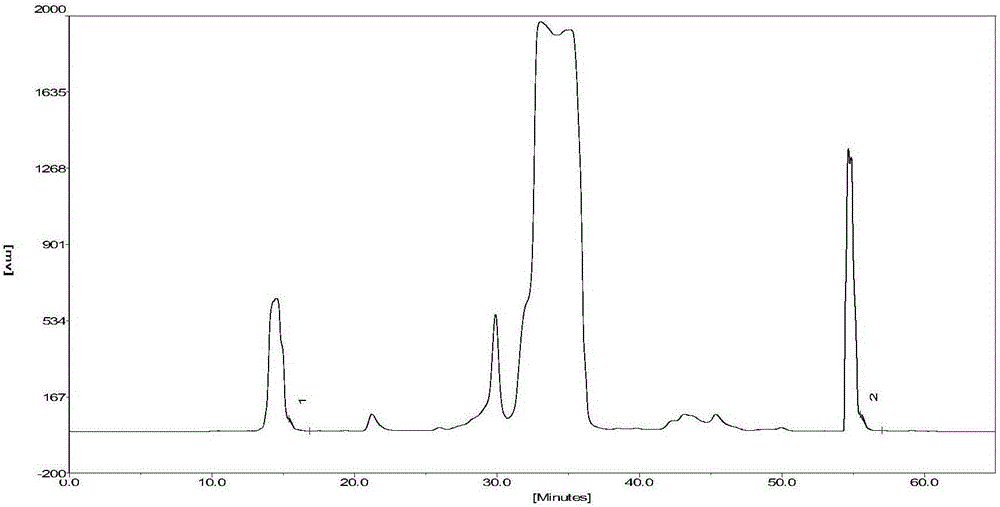
[0067] The crude sample is an acylated insulin solution prepared by one-step reaction of B30-removed recombinant human insulin and palmitic acid N-succinimidyl ester, the method is the same as in Preliminary Example 1; the chromatographic purification method adopts advanced silica gel carrier packing ( ) is carried out on a semi-preparative chromatographic column (50mm × 250mm), and the filling pressure is 7-10MPa; the mobile phase A phase for elution: 20mmol / L phosphate buffer, adjust disodium hydrogen phosphate and dihydrogen phosphate in the buffer The ratio of sodium, so that the pH of phase A is 7.0, and the mobile phase B for elution: acetonitrile-water (mixed by volume ratio 9:1); keep the column temperature at 20-25 °C; the elution flow rate is 82.5ml / min; The detection wavelength is 280nm.
[0068] The specific steps are the same as in Example 1. The isocrat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com