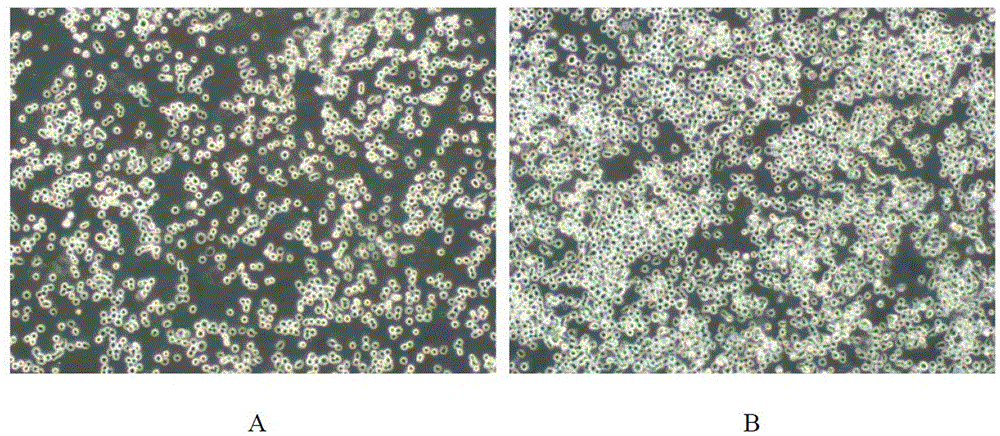
Low serum medium for full-suspending culture of MDCK cells
A medium and full suspension technology, applied in the direction of animal cells, culture process, tissue culture, etc., can solve the problems of low density and no medium, and achieve the effect of increasing cell density, low price, and increasing cell density
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] The formula of the low-serum medium of the whole suspension culture MDCK cell of the present invention is as follows, the default unit: mg / L; wherein, the underlined part is the DMEM / F12 medium composition:
[0050] 1) Inorganic salts and trace elements:
[0051] Calcium Chloride CaCl 2 116.6
[0052] Potassium chloride KCl311.8
[0053] Magnesium Sulfate MgSO 4 48.84
[0054] Anhydrous disodium hydrogen phosphate Na 2 HPO 4 71.02
[0055] Sodium dihydrogen phosphate NaH 2 PO 4 -H 2 O62.5
[0056] Magnesium Chloride MgCl 2 28.64
[0057] Copper Sulfate CuSO 4 ·5H 2 O0.0013
[0058] Iron nitrate Fe(NO 3 ) 3 ·9H 2 O0.05
[0059] Ferrous Sulfate FeSO 4 ·7H 2 O0.417
[0060] Zinc sulfate ZnSO 4 ·7H 2 O0.432
[0061] Sodium pyruvate C 3 H 3 NaO 3 55
[0062] Nickel chloride NiCl 2 ·6H 2 O0.000195
[0063] Ammonium heptamolybdate (NH 4 ) 6 Mo 7 o 24 .4H 2 ...
Embodiment 2
[0123] The difference between this embodiment and embodiment 1 is:
[0124] In culture medium:
[0125] Sodium selenite 0.004mg / L
[0126] Sodium chloride NaCl6500mg / L
[0127] Vitamin A0.1mg / L
[0128]Vitamin E0.1mg / L
[0129] Human transferrin 5mg / L
[0130] Recombinant insulin 20mg / L
[0131] Glutathione 0.3mg / L
[0132] Vegetable protein hydrolyzate 2250mg / L
[0133] Recombinant human serum albumin 200mg / L
[0134] PF68800mg / L.
[0135] The remaining components of the culture medium and the preparation method of the culture medium are the same as in Example 1.
Embodiment 3
[0137] The difference between this embodiment and embodiment 1 is:
[0138] In culture medium:
[0139] Sodium selenite 0.003mg / L
[0140] Sodium chloride NaCl5000mg / L
[0141] Vitamin A0.2mg / L
[0142] Vitamin E0.08mg / L
[0143] Human transferrin 10mg / L
[0144] Recombinant insulin 15mg / L
[0145] Glutathione 0.1mg / L
[0146] Vegetable protein hydrolyzate 2500mg / L
[0147] Recombinant human serum albumin 300mg / L
[0148] PF681200mg / L.
[0149] The remaining components of the culture medium and the preparation method of the culture medium are the same as in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Cell density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com