Skeletal muscle stem cell serum-free medium and preparation method and application thereof
A serum-free medium, stem cell technology, applied in cell culture medium, cell culture active agent, bone/connective tissue cells, etc., can solve the problems of difficulty in purifying skeletal muscle stem cells and low expansion efficiency, and improve the expansion efficiency. and the effect of high purity, high amplification efficiency and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1 Preparation of serum-free medium for human skeletal muscle stem cells
[0038] Cell basal medium (DMEM: F12 (1:1) dry powder, product number: SH30004, purchased from Thermo Company), cytokines (purchased from PEPROTECH Company), metrophin (purchased from Sigma Company, the purity is more than 98%), Human Recombinant Insulin (purchased from Sigma, above 98% purity), Chondroitin 6-sulfate (purchased from Hainan Culuwa Pharmaceutical Co., Ltd.), Vitamin C (purchased from Sigma, above 98% purity), serum replacement (trade name "Ultroser G", brand Lonza; also available from Crescent Chemical Co., Inc.), NaHCO 3 (purity of 98% or more purchased from Sigma) and amino acids (purity of 98% or more purchased from Sigma), transferrin (purity of 98% or more purchased from Sigma), L-glutamine (purity of 98% or more purchased from Sigma) from Sigma company, the purity is more than 98%), vitamin B6 (purchased from Sigma company, the purity is more than 98%), thiamine hydroc...
experiment example 1
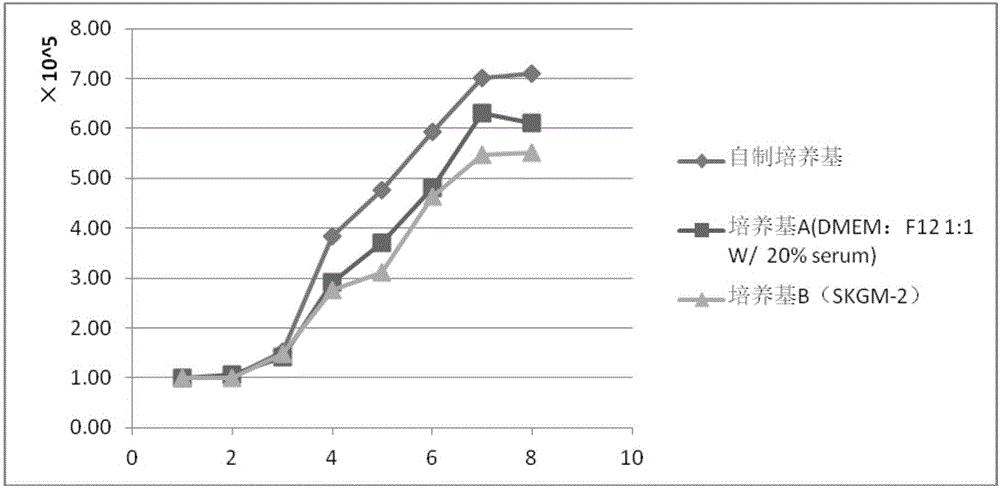
[0047] Experimental Example 1 Comparison of growth curves of skeletal muscle stem cells in three media
[0048] The three media used in this experiment are serum-free media prepared in Example 1 of the present invention (hereinafter referred to as self-made media), DMEM:F12 1:1 media with 20% fetal bovine serum (hereinafter referred to as media A) , purchased from Lonza company's skeletal muscle stem cell special low serum medium SKGM-2 (hereinafter referred to as medium B).
[0049] 1. Isolation and purification of skeletal muscle stem cells
[0050] (1) Surgical biopsy Take 2-5 g of rectus femoris or deltoid muscle voluntarily donated by young healthy people aseptically, transfer it into a 50 ml centrifuge tube containing 20 ml of PBS containing 1% penicillin and streptomycin, and transport it to the laboratory at 4°C.
[0051] (2) Take out the muscle tissue from the 50ml centrifuge tube, put it into a sterile dish, remove the fat and connective tissue, and cut it into abou...
experiment example 2
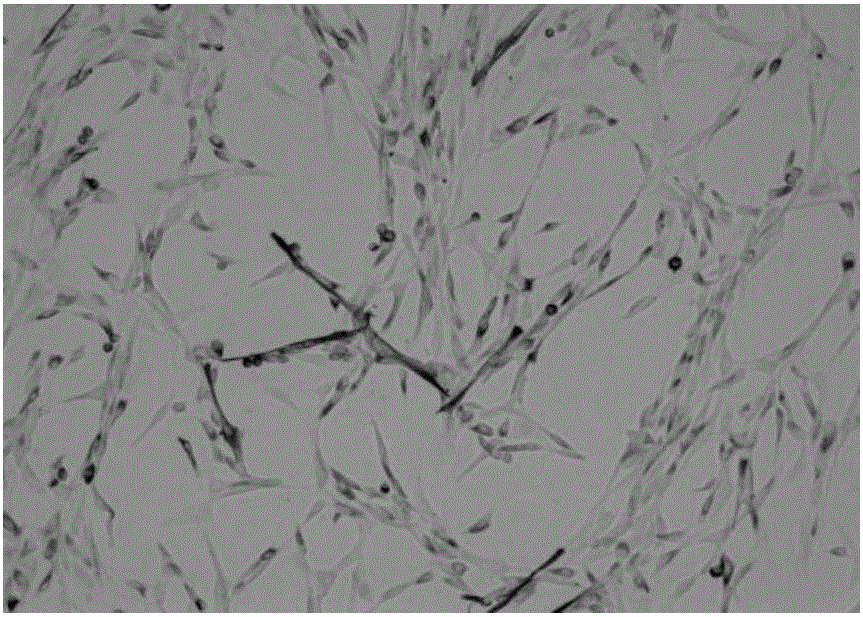
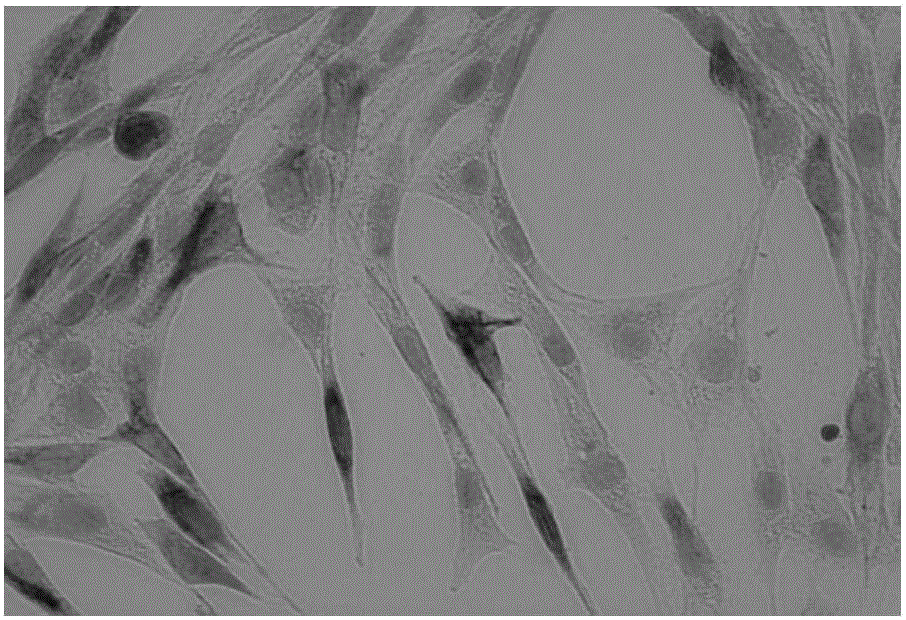
[0067] Experimental example 2 Purity identification and differentiation ability evaluation of skeletal muscle stem cells
[0068] Desmin antibody, immunohistochemical kits and auxiliary reagents were purchased from Wuhan Boster Company; PBS was phosphate buffered saline with pH 7.2-7.4; SABC reagent, DAB chromogenic reagent, DAB chromogenic reagent (purchased from Wuhan Boster Company) ).
[0069] 1. Digest P20 skeletal muscle stem cells and collect them with homemade medium at 2×10 5 The density of cells / well was added to a 1:10 dilution of polylysine-coated 6-well plate for culture. When the cells were more than 90% of the 6-well plate, it was used for immunohistochemical experiments.
[0070] 2. Wash the cells 3 times with PBS solution, 2 min / time.
[0071] 3. Add 1ml / well of 4% paraformaldehyde and fix for 30min at room temperature.
[0072] 4. Wash the cells 3 times with PBS solution, 2 min / time.
[0073] 5. Add 0.5% triton x-100 1ml / well for 10min to enhance cell per...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com