Animal protein-free immune cell serum-free medium and using method thereof
A serum-free culture medium, immune cell technology, applied in the field of in vitro culture of immune cells, can solve the problems of poor cell proliferation efficiency and biological efficacy, pollution, serum quality differences, etc., to improve cell proliferation efficiency and biological efficacy, The effect of good tumor killing rate and controllable quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Use triple-distilled water as a solvent, and dissolve amino acids, vitamins, salts, organic matter and protein to prepare a medium. To increase the solubility of organic matter, add co-solvent Tween 80, use 0.1mol / L hydrochloric acid and 0.1mol / L Sodium hydroxide is used to adjust the pH value so that the pH value of the medium is 6.8-7.2. The culture medium was sterilized twice through a 0.22um filter membrane, and stored in a refrigerator at 4°C in the dark.
[0031] Based on 1L of the prepared medium, its components contain the following concentrations of components:
[0032] Amino acid composition: glycine concentration 18mg / L, L-arginine concentration 190mg / L, L-asparagine 45mg / L, L-aspartic acid 20mg / L, L-cystine hydrochloride 60mg / L, L-glutamic acid 15mg / L, L-histidine 25mg / L, L-hydroxyproline 15mg / L, L-isoleucine 130mg / L, L-leucine 80mg / L, L-hydrochloric acid Lysine 100mg / L, L-methionine 25mg / L, L-phenylalanine 35mg / L, L-proline 25mg / L, L-serine 45mg / L, L-thre...
Embodiment 2
[0039] The culture medium prepared in embodiment 1 is carried out quality test
[0040] 1) Sterility test
[0041] Using the plate method for detection, take 1 sheep blood agar plate and 1 Saburo agar plate, and equilibrate to room temperature. Take the culture medium prepared in Example 1 as the sample, centrifuge at 4000rpm for 15min, draw the sample from the bottom of the sample tube, 100 μl per plate, and streak with an inoculation needle. Put the plate into a 37°C incubator, and after culturing for 48 hours, the observation result was negative, and the sample was qualified.
[0042] 2) Endotoxin detection
[0043] Use the gel method to detect, take the culture medium prepared in Example 1 as the sample after dilution, add the same negative control, positive control, and test sample positive control respectively in the Limulus reagent after sterility testing water reconstitution, each Parallel two tubes. The result is that the negative control tube is negative, the pos...
Embodiment 3
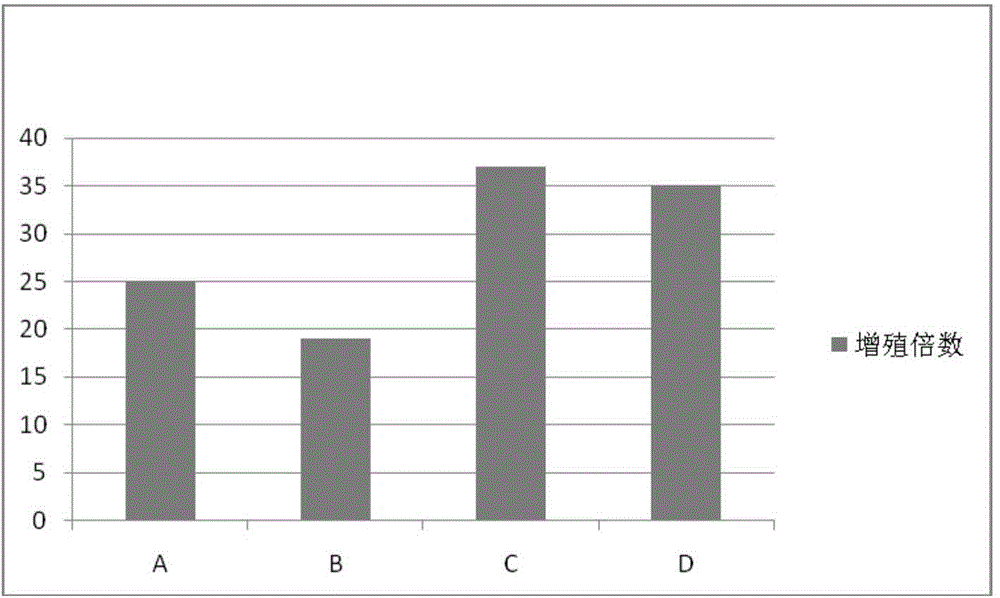
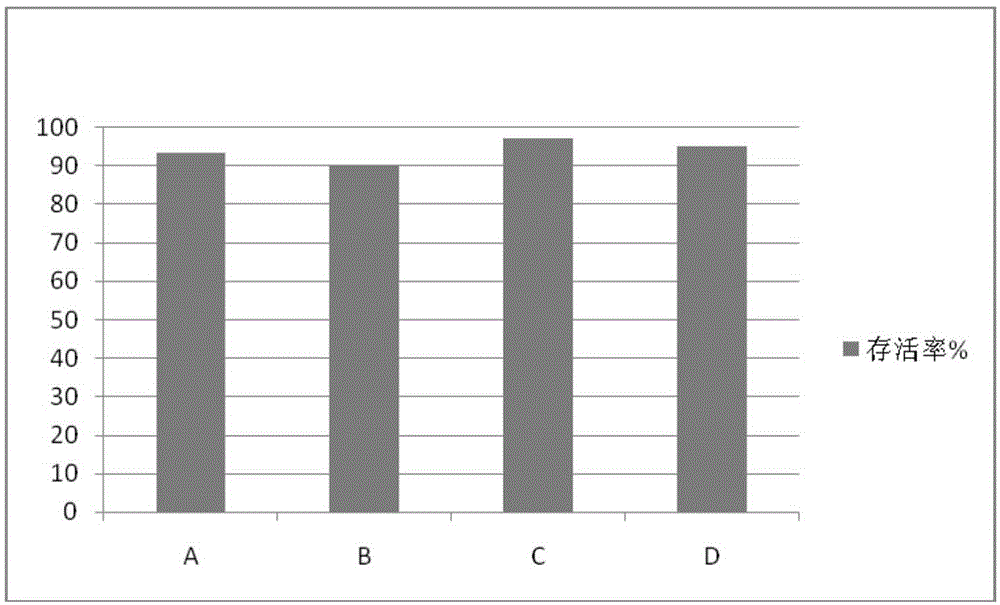
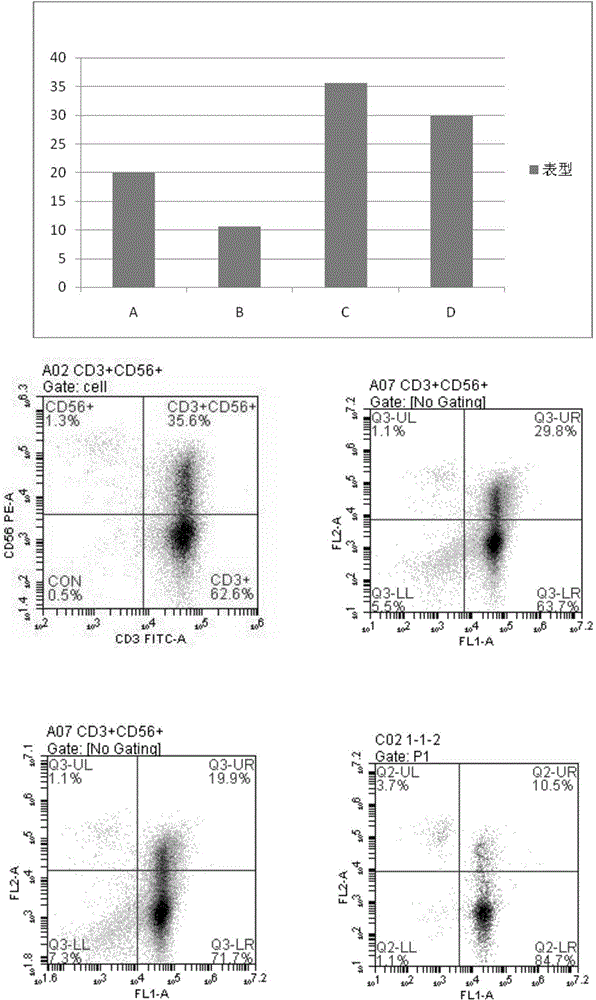
[0047] Use lymphocyte separation medium density gradient centrifugation to separate peripheral blood mononuclear cells, or to revive frozen mononuclear cells. Centrifuge at 500 g of normal saline for 5 minutes, and remove the supernatant. Count the cells, and use the culture medium prepared in Example 1 to make a concentration of 1 × 10 6 cells / mL of cell suspension. Add 2 mL of culture medium cell suspension to each well of 6-well plate, with 3 parallel wells in each group. On the first day, 1000IU / mL INF-r was added and cultured in a carbon dioxide incubator. After 24 hours, 50ng / mL CD3 monoclonal antibody and 500IU / mL IL-2 were added. Every 48 hours thereafter, supplement the culture medium and 500IU / mL IL-2, and count to 1×10 6 cells / mL concentration. Cell morphology was observed after co-cultivation for 18 days. The culture result is: the shape of the cells is polygonal, the shape is plump, and the growth is good.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com