Preparation method of muramyl dipeptide-anti-CD20 immune conjugate and application thereof
A technology of immunoconjugates and muramyl dipeptide, which is applied in the direction of anti-animal/human immunoglobulin, anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, dipeptide components, etc., and can solve the problem of Difficulty in identifying or eliminating tumor cells, immune function impairment, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
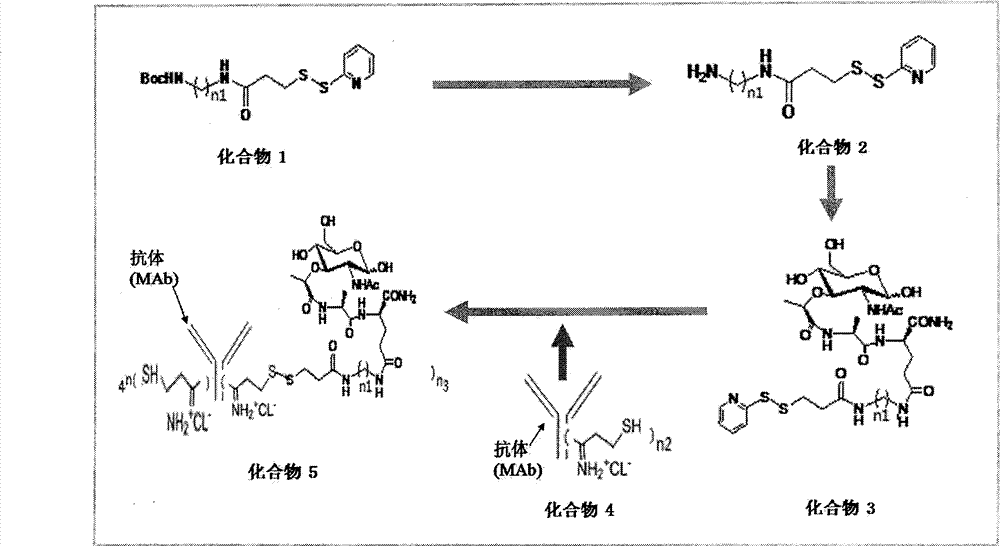
[0012] [Example 1] Preparation of Compounds 1-5
[0013] Preparation of Compound 1
[0014] Take, for example, a linker arm containing three carbon atoms. Under argon protection, in a 10ml flask, add Boc-protected linker N-Boc-1,3-propanediamine hydrochloride (20mg, 0.12mmol, CAS number: 127346-48-9, Sigma-Aldrich company) , dissolved with 3ml dimethylformamide, added excess triethylamine, and then added N-succinimide-3-(2-pyridylthio)propionate (SPDP, 74mg, 0.24mmol, CAS No.: 68181 -17-9, Sigma-Aldrich Company), stirred at room temperature for 1 h, and the spot plate detection reaction was completed, then separated through a silica gel column (ethyl acetate: petroleum ether = 1: 1), and evaporated to dryness in vacuo to obtain the product compound 1 (19.8 mg). ESI-MS m / z 372.1[M+H] + .
[0015] Preparation of Compound 2
[0016] Under the protection of argon, in a 10ml reaction flask, add compound 1 (10mg, 0.02mmol), add 1.5ml of dichloromethane, stir in ice bath for 10...
Embodiment 2
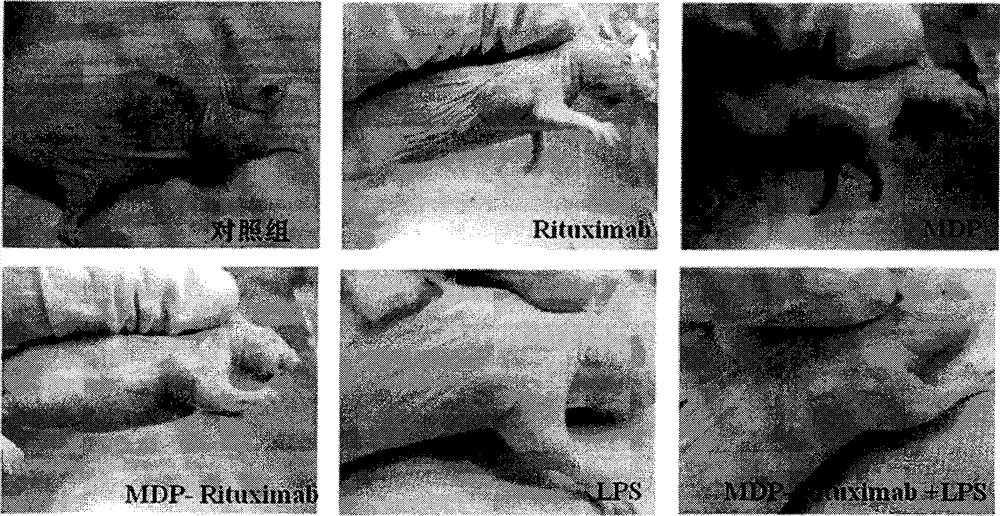
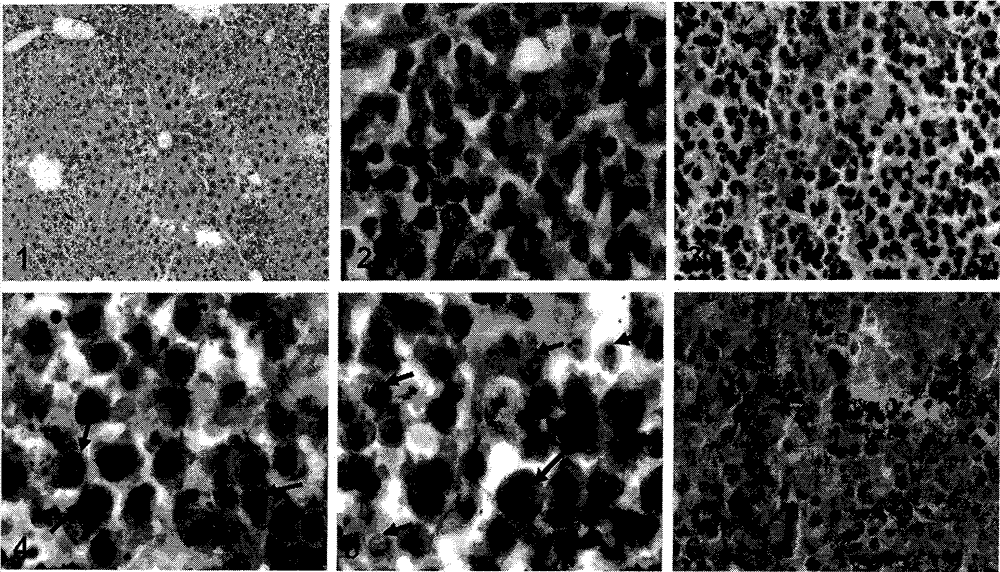
[0029] [Example 2] Test of immune activity
[0030] 1. In vitro immune activity
[0031] 1.1 Test method
[0032] Preparation of the test sample solution: the test sample is the compound 5 synthesized in the above-mentioned Example 1 and unconjugated anti-CD20 monoclonal antibody, MDP, lipopolysaccharide (LPS, Escherichia coli 0128: B12, Sigma-Aldrich Company). Accurately weigh an appropriate amount of sample, and use RPMI1640 complete culture medium to prepare a solution of the required concentration for activity testing.
[0033] The present invention adopts the method of indirect flow cytometry combined with antibody-antigen specific binding to test and evaluate the binding activity of the tested sample to CD20+ lymphoma cells (Raji cells, ≥90%). The anti-CD20 monoclonal antibody specifically binds to the CD20 antigen of lymphoma cells, FITC-labeled non-specific secondary antibody is added, incubated in the dark, and the fluorescence intensity is detected by flow cytometr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com