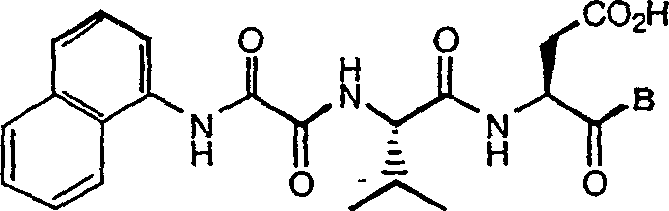
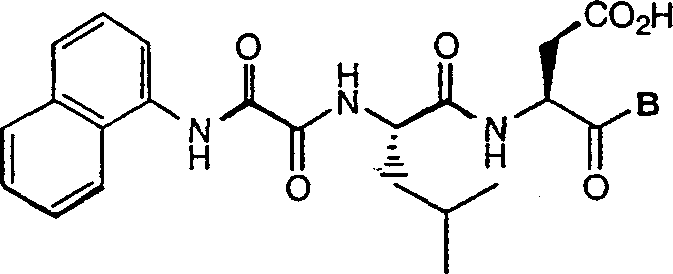
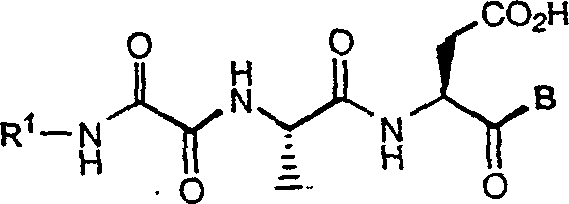
C-terminal modified oxamyl dipeptides as inhibitors of the ICE/ced-3 family of cystenine proteases
An amino acid, CH2OCO technology, used in the treatment of autoimmune diseases, in the field of pharmaceutical compositions containing these compounds, can solve problems such as obstacles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0145] In the following examples, the proton NMR spectrum is measured at 300 MHz; the chemical shift value is reported for the low magnetic field of the internal standard tetramethylsilane. Preparation Example 1 (3S)-Amino-4-oxobutyric acid tert-butyl semicarbazone p-toluenesulfonate Part A: N-(benzyloxycarbonyl)-L-(N'-methyl-N'-methoxy) Asparagine β-tert-Butyl Ester
[0146] In 0℃ (ice bath) and nitrogen atmosphere, into N-(benzyloxycarbonyl)-L-aspartic acid β-tert-butyl ester (14.65g, 45.3mmol, Bachem) in dichloromethane (150mL) Add 1-hydroxybenzotriazole hydrate (7.29g, 47.6mmol, Aldrich), then add 1-ethyl-3-(3',3'-dimethyl-1'-aminopropyl) carbodiimide Amine hydrochloride (9.55 g, 49.8 mmol, Sigma). After stirring for 15 minutes at 0°C, N,O-dimethylhydroxylamine hydrochloride (5.10g, 52.3mmol, Aldrich) and N-methylmorphine (5.8ml, 53mmol, Aldrich) were added. After 3 hours, the mixture was allowed to warm to room temperature, and then stirred at room temperature for 16 hours. ...
preparation example 2
[0152] By conversion to the corresponding Mosher amide [1.05 equivalents (R)-(-)-α-methoxy-α-(trifluoromethyl)phenylacetyl chloride, 2.1 equivalents diisopropylethylamine (i-Pr2NEt) Dichloromethane solution, room temperature, 30 minutes], the optical purity of the product was detected. The target product has a double peak at 7.13 ppm (1H, d, J=2.4 Hz, CH=N), and the corresponding signal of its diastereomer is at 7.07 ppm. The optical purity of the title compound obtained by the above method is usually >95:5. Preparation Example 2 (3S)-(9-Fluorenylmethoxycarbonyl)amino-4-oxobutyrate tert-butyl semicarbazonyl-4-[2'-(4-ethyl-phenoxyacetic acid)] Preparation part A: 4-[2'-(N-tert-butoxycarbonyl)aminoethyl]phenoxyacetic acid, methyl ester
[0153] At room temperature and under a nitrogen atmosphere, to a suspension of 4-hydroxyphenethylamine (7.00g, 51.1mmol, Aldrich) in anhydrous dimethylformamide (50ml) was added di-tert-butyl dicarbonate (11.0g, 50.5mmol). After stirring at room te...
preparation example 3
[0161] Basically in accordance with the method of Thornberry et al. (Nature, 356:768:774 (1992)) and Nicholson et al. (Nature, 376:37-43 (1995)) (incorporated herein for reference), in a 96-well microtiter plate Recombinant ICE and CPP32 enzymes were used in the luciferase assay to determine the activity of the compound of formula 1. The substrate used for ICE analysis is acetyl-Tyr-Val-Ala-Asp-amino-4-methylcoumarin (AMC), and the substrate used for CPP32, Mch2, Mch3 and Mch5 analysis is acetyl-Asp -Glu-Val-Asp-amino-4-methylcoumarin. At room temperature, the enzyme reaction was repeated (in duplicate) in ICE buffer (25 mM HEPES, 1 mM EDTA, 0.1% CHAPS, 10% sucrose, pH 7.5) containing 2 mM DTT. The analysis is performed by mixing the following ingredients:
[0162] 50μl ICE, Mch2, Mch5, CPP32 (concentrations of 18.8, 38, 8.1 and 0.153nM) or Mch3 (18.8, 38, 8.1 and 0.153nM) in 50μl of ICE, Mch2, Mch5, CPP32 in ICE buffer containing 8.0 (ICE, Mch2, Mch3, CPP32) or 20 (Mch5) mM DTT U...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com