Method for rapidly detecting virus titer of hepatitis A vaccine based on PMA-qRT-PCR method
A hepatitis A, detection method technology, applied in biochemical equipment and methods, microbial determination/inspection, resistance to vector-borne diseases, etc., can solve the problems of long detection cycle, heavy workload, complicated operation, etc. Test period, good linear effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
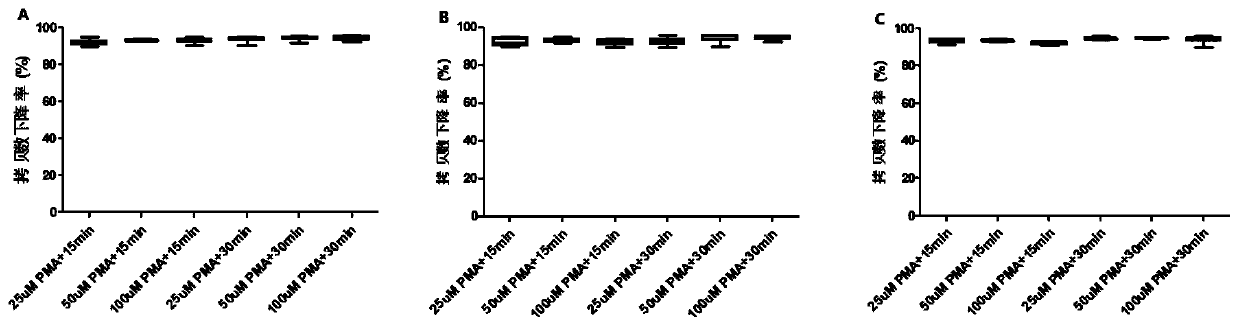
[0040] Embodiment 1 investigates the influence of the concentration of PMA and photolysis time on detection result
[0041] Human diploid cell KMB17 was cultured to a monolayer in a cell culture flask, then poured off the excess medium, added PBS buffer to the cells to wash the cell surface, then discarded the excess PBS buffer, and then added trypsin to digest the cells , followed by diluting the cells with MEM medium to 0.5-1.0×10 6 a / ml;
[0042] Take the hepatitis A vaccine harvest liquid or finished product as the sample to be tested;
[0043] Add 1.0ml of diluted cells into a 1.5ml centrifuge tube, discard the supernatant after centrifugation;
[0044] Add 300 μl / tube of the sample to be tested to the cells in step (3), resuspend the cells, and incubate with shaking at 37°C for 2 hours;
[0045] Add PMA respectively, so that the final concentration of PMA is 25, 50, 100 μM, mix well, and incubate at 4°C for 30 minutes in the dark;
[0046] Freeze and thaw the broken ...
Embodiment 2
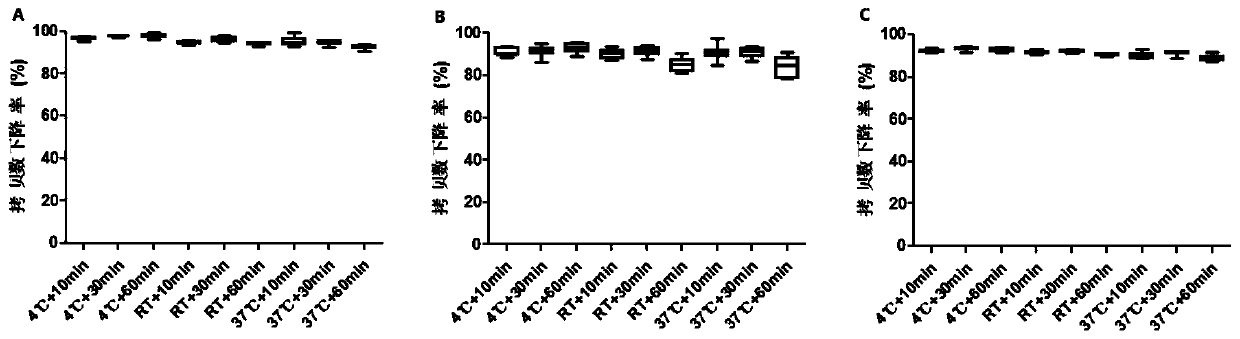
[0051] Embodiment 2 investigates the influence of incubation temperature and time of PMA on detection result
[0052] The same method as in Example 1 was carried out, except that after adding 50 μM PMA, the freeze-dried live attenuated hepatitis A vaccine was tested under the conditions of incubation temperature 4°C, room temperature, and 37°C, and incubation time 10, 30, and 60 minutes, respectively. 10 consecutive tests.
[0053] The result is as figure 2 As shown, the copy number decrease rate of PMA under the condition of 4°C dark-proof incubation was significantly higher than that at room temperature and 37°C (P<0.05). Among them, the average copy number decline rates of the 9 experimental groups of P1-P4 primers were 92.19%, 93.46%, 92.91%, 91.60%, 91.91%, 90.48%, 90.43%, 91.46% and 90.92%. Among them, the 4°C+30min group had the highest copy number decline rate (93.46%), which was significantly higher than that of the 4°C+10min group (P=0.019); no significant differe...
Embodiment 3
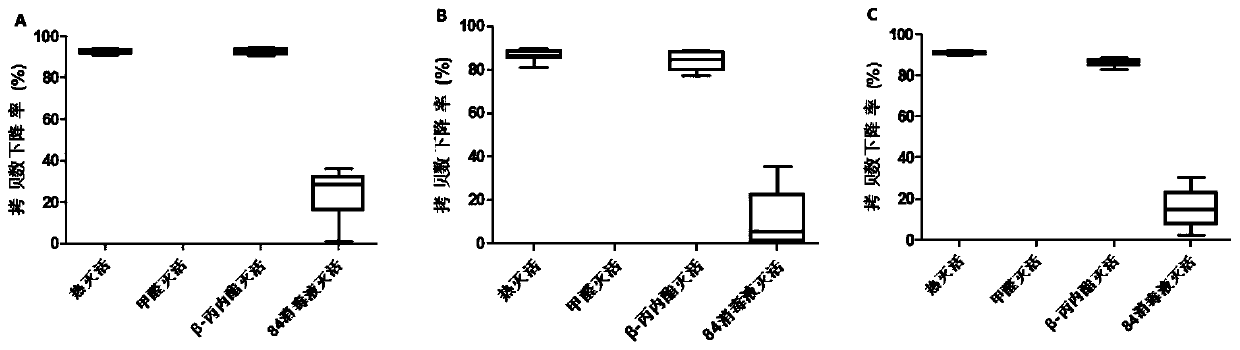
[0054] Embodiment 3 investigates the impact of different inactivation methods of hepatitis A vaccine on detection results
[0055] Carry out the same method as Example 2, the difference is that the following conditions are used to process the hepatitis A live attenuated vaccine: ① heat inactivation: incubate at 70°C for 5 minutes; ② formaldehyde inactivation: the volume ratio of vaccine sample to inactivator is 1:1250 For inactivation, incubate at 37°C for 12 days; ③β-propiolactone inactivation: inactivate according to the volume ratio of vaccine sample to inactivator 1:2000, and incubate at 4°C for 24h; ④84 disinfectant inactivation: according to the volume ratio of vaccine sample and inactivator Inactivation was carried out with a volume ratio of 1:90, and incubated at room temperature for 20 min. Six consecutive tests were performed in each group. The result is as image 3 As shown, wherein, P1-P4 primers detect heat inactivation, β-propiolactone inactivation and 84 disin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Titer | aaaaa | aaaaa |
| Titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com