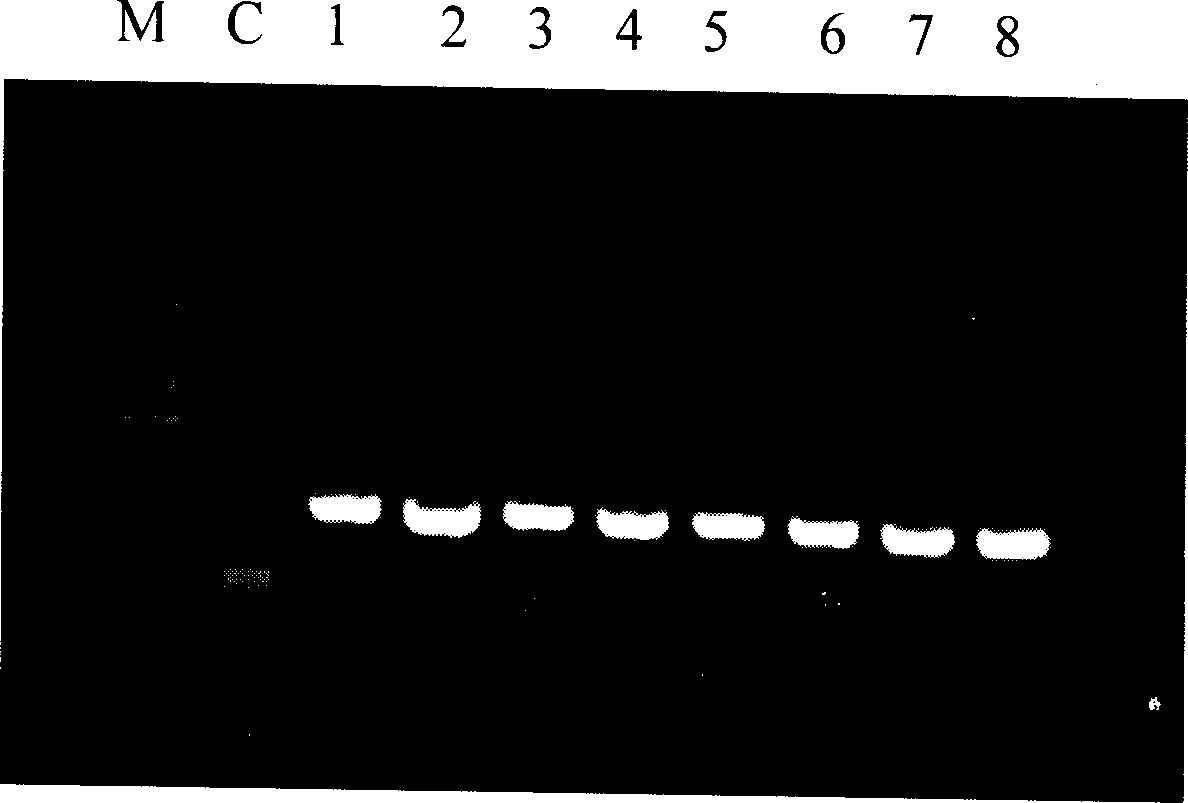
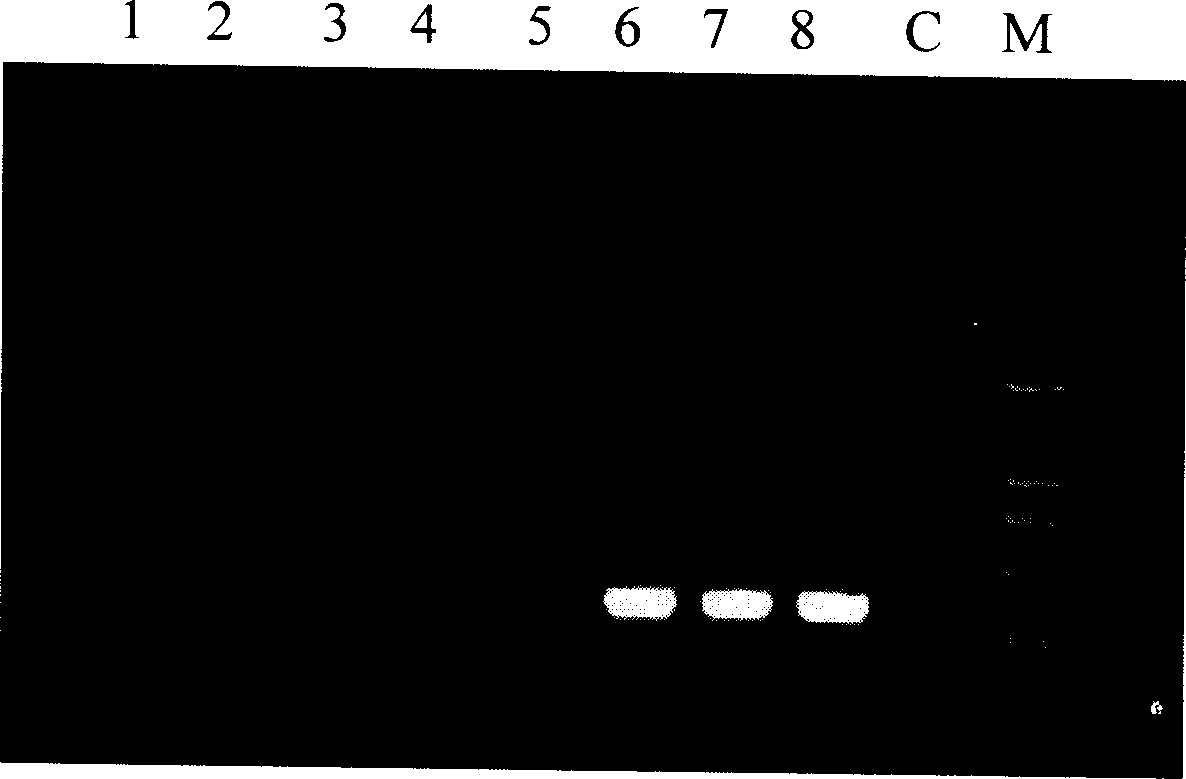
Fast and sensitive method of detecting virus infection titer of attenuated live hepatitis A vaccine
A live attenuated vaccine and sensitive detection technology, applied in biochemical equipment and methods, microbe measurement/inspection, and resistance to vector-borne diseases, etc., can solve the problems of complicated steps, long time-consuming, long vaccine storage period, etc., to achieve The effect of overcoming complicated steps, shortening the detection cycle, and reducing the probability of contamination
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] 1. Virus culture
[0079] The KMB17 cells were divided into small square bottles, and after two days of culture, they grew into a fresh monolayer; the hepatitis A live attenuated vaccine sample Ref was serially diluted 10 times, and 10 -5 , 10 -6 , 10 -7 and 10 -8 Dilutions were inoculated on KMB17 cells, 4 bottles of cells were inoculated for each dilution, 1.0ml of virus solution was inoculated in each bottle of cells, after adsorption for 2 hours, maintenance solution was added, and cultured at 35°C. The other two bottles of cells were added with 1ml of phosphate buffered saline as negative control.
[0080] 2. Extraction of intracellular hepatitis A virus RNA
[0081] After culturing for 8 days, drain the maintenance solution in the culture bottle, add 2.5ml TRIzol reagent to each bottle for lysis, and divide the uniform lysate into two 1.5ml tubes, one tube is used for subsequent experiments immediately, and the other tube is stored at -70 ℃ for standby, and t...
Embodiment 2
[0125] 1,2,3 and 4 steps are the same as embodiment 1;
[0126] 5. Detection of negative-strand RNA intermediates of hepatitis A virus
[0127] (1), reverse transcription reaction
[0128] Add the following reagents in sequence to a nuclease-free 0.5ml centrifuge tube:
[0129] 10× reverse transcription buffer 5μl
[0130] dNTP mix (3mmol / L) 4μl
[0131] Forward primer N1 (5~60μmol / L) 2μl
[0132] RNase inhibitor (40u / l) 2μl
[0133] Reverse transcriptase (4u / μl) 2μl
[0134] HAV RNA 30μl
[0135] Nuclease-free water Make up to a total reaction volume of 50 μl
[0136] React at 42°C for 120 minutes; boil the obtained reverse transcription reaction product for 75 minutes;
[0137] (2), the first round of polymerase chain reaction
[0138] Add the following reagents in sequence to a nuclease-free 0.5ml centrifuge tube:
[0139] 10×PCR buffer 15μl
[0140] dNTP mix (3mmol / L) 4μl
[0141] Reverse primer N2 (5~60μmol / L) 2μl
[0142] Forward primer M1 (5~60μmol / L) 2μl ...
Embodiment 3
[0159] 1,2,3 and 4 steps are the same as embodiment 1;
[0160] 5. Detection of negative-strand RNA intermediates of hepatitis A virus
[0161] (1), reverse transcription reaction
[0162] Add the following reagents in sequence to a nuclease-free 0.5ml centrifuge tube:
[0163] 10× reverse transcription buffer 3.5μl
[0164] dNTP mix (8mmol / L) 2μl
[0165] Forward primer N1 (5~60μmol / L) 1μl
[0166] RNase inhibitor (40u / μl) 1μl
[0167] Reverse transcriptase (4u / μl) 2μl
[0168] HAV RNA 15μl
[0169] Nuclease-free water Make up to a total reaction volume of 35 μl
[0170] React at 40°C for 100 minutes; boil the obtained reverse transcription reaction product for 55 minutes;
[0171] (2), the first round of polymerase chain reaction
[0172] Add the following reagents in sequence to a nuclease-free 0.5ml centrifuge tube:
[0173] 10×PCR buffer 12μl
[0174] dNTP mix (6mmol / L) 4μl
[0175] Reverse primer N2 (5~60μmol / L) 3μl
[0176] Forward primer M1 (5~60μmol / L) 3μ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Titer | aaaaa | aaaaa |
| Titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com