Improved lyophilized live attenuated hepatitis a vaccine stabilizer, vaccine finished product, vaccine semi-finished product and preparation method of vaccine finished product and vaccine semi-finished product
A technology for attenuated live vaccines and hepatitis A, applied in biochemical equipment and methods, antiviral agents, freeze-dried transportation, etc., can solve the problems of slow product dissolution, increased production costs, and increased dosage of excipients, and achieve prevention Effects of spillover of live virus, reduction of production costs, and increase in drug use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0052]The preparation method of the finished product of the improved freeze-dried hepatitis A attenuated vaccine provided by the invention mainly comprises the following steps:
[0053] Step 1. After the hepatitis A virus harvest liquid is prepared by the attenuated strain of hepatitis A virus seed L-A-1 with the preservation number of CCTCC No.V92004, the virus harvest is subjected to cell disruption and purification after extraction to obtain a vaccine stock solution.
[0054] Step 2, prepare the vaccine stabilizer, formula and concentration (final concentration is the mass volume fraction, g / mL) are as follows (the concentration of its components is 2 times of the corresponding improved stabilizer component concentration): Trehalose: 4% -12%, Monosodium Glutamate: 0.2%-2.0%, Arginine: 0.1%-0.6%, Urea: 0.2%-1.6%, Vitamin C: 0%-1.0%, Dextran 40: 0%-4.0% , sorbitol: 0%-1.0%, mannitol: 0.1%-1.0%; weigh the drug according to the above formula, dissolve it in water for injection...
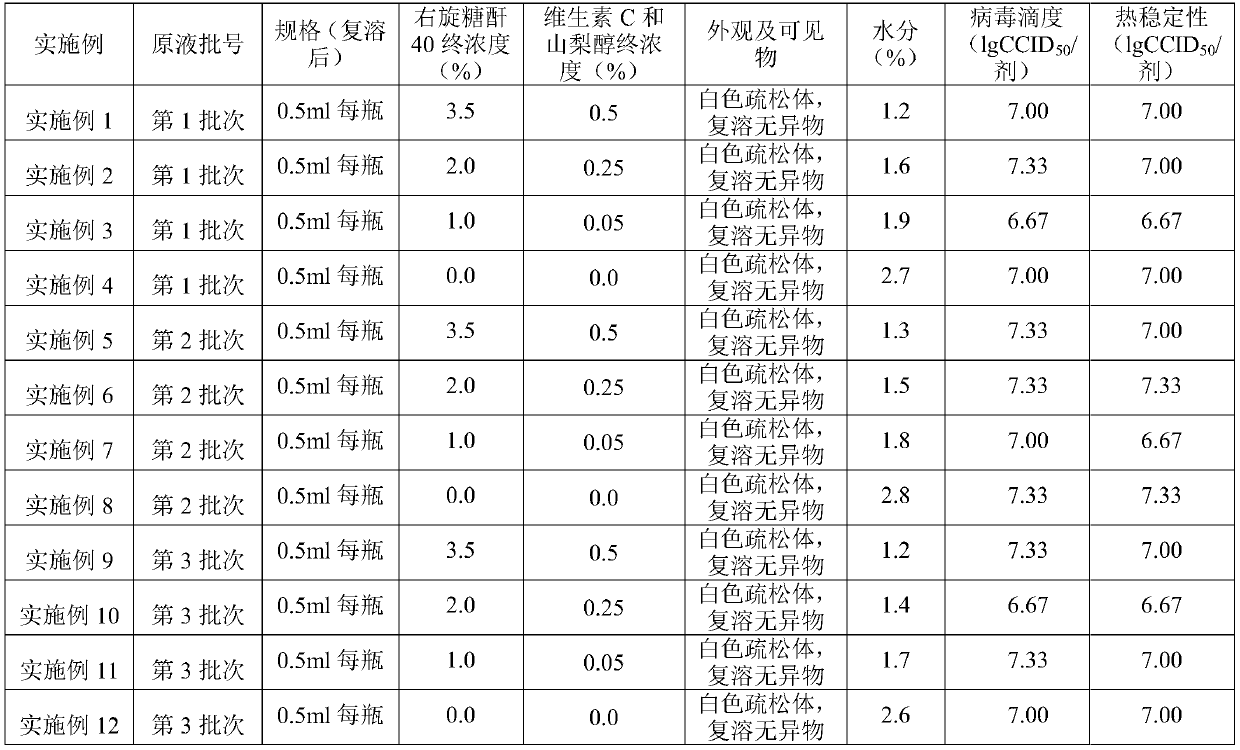
Embodiment 1~12
[0071] According to the prior art, 3 batches of vaccine stocks with the same conditions were prepared as experimental groups for parallel experiments, namely: the 1st batch, the 2nd batch, and the 3rd batch; Store after preparation.
[0072] Prepare 4 batches of stabilizers as the experimental group of parallel experiments, the first batch of vaccine stabilizer: dextran 40 concentration 7.0%, vitamin C concentration 1.0%, sorbitol concentration 1.0%; the second batch of vaccine stabilizer: dextran 40 concentration 4.0%, vitamin C concentration 0.5%, sorbitol concentration 0.5%; the 2nd batch vaccine stabilizer: dextran 40 concentration 2.0%, vitamin C concentration 0.1%, sorbitol concentration 0.1%); the 4th batch vaccine stabilizer: The concentration of dextran 40 is 0.0%, the concentration of vitamin C is 0.0%, and the concentration of sorbitol is 0.0%); it is prepared by the vaccine second room of Changchun Institute of Biological Products Co., Ltd.
[0073] Preparation of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Titer | aaaaa | aaaaa |
| Titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com