HBsAg, technology for preparing HBsAg through expression by virtue of recombinant saccharymyces cerevisiae, separation and purification technology for HBsAg as well as hepatitis B vaccine
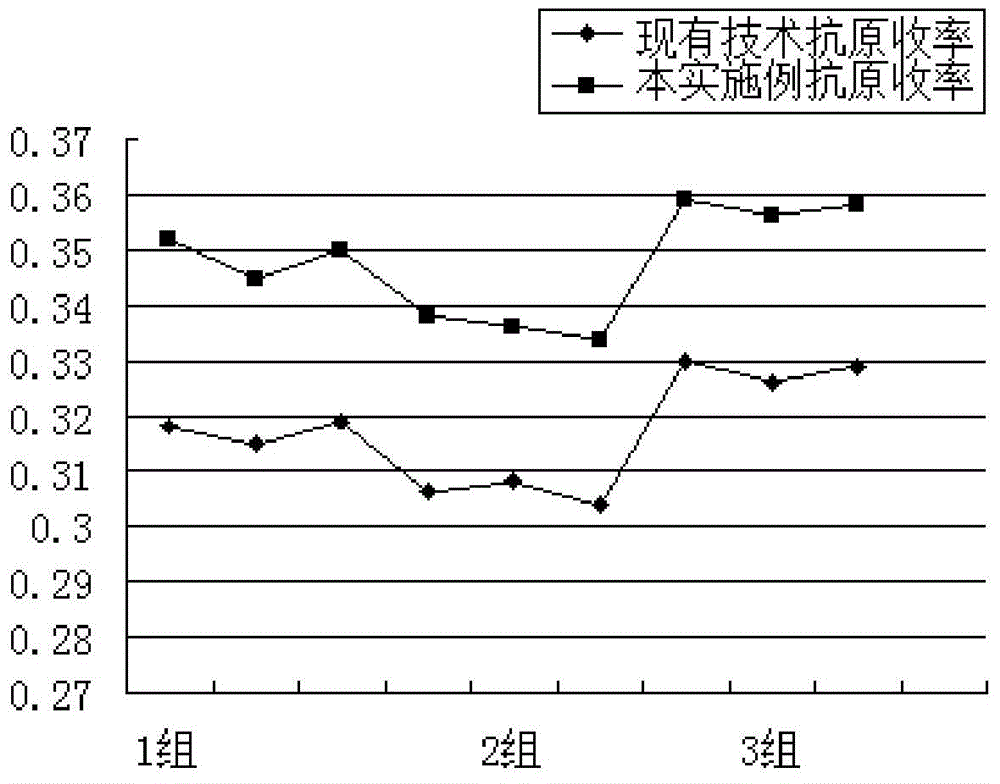
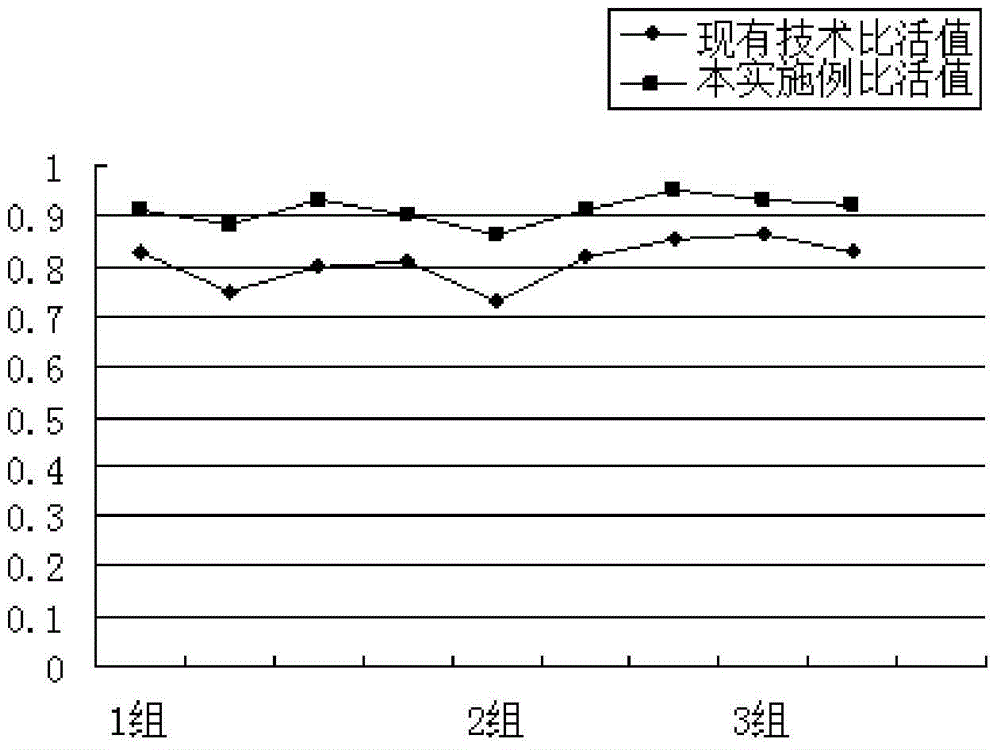
A technology for the separation and purification of recombinant Saccharomyces cerevisiae, which is applied in chemical instruments and methods, biochemical equipment and methods, and medical preparations containing active ingredients, etc. It can solve the problem of stock solution yield and quality, short half-life, and increase the difficulty of purification, etc. problems, achieve high economic and social benefits, improve specific activity, and increase yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0034] Refer below figure 1 Describe in detail the first embodiment of the separation and purification process of recombinant Saccharomyces cerevisiae expressing HBsAg provided by the present invention; this embodiment realizes a separation and purification process of recombinant Saccharomyces cerevisiae expressing HBsAg, which mainly includes the following steps:
[0035] In the cell breaking step, the yeast cells extracted after fermentation are fed into a high-pressure homogenizer, and the cells are broken under a pressure of 15000±1500PSIG;
[0036] In the purification step, the crushed material liquid is purified to prepare the HBsAg stock solution.
[0037] And, crucially, after the cell disruption step and before the purification step, it also includes:
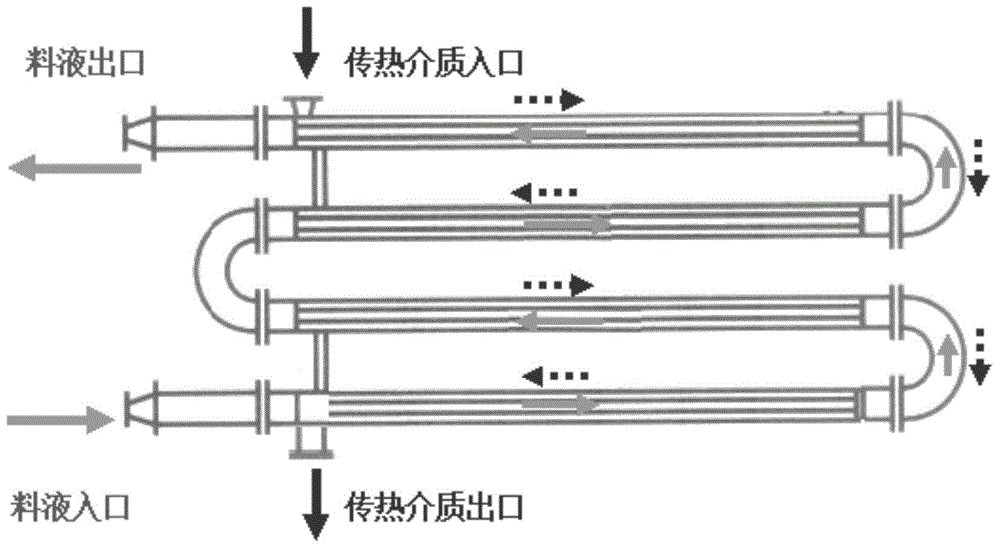
[0038] In the feed liquid heating step, the crushed feed liquid is heated to raise the temperature to 50-80°C, and then lowered to 2-8°C.
[0039] During the specific implementation, in the step of heating the feed l...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com