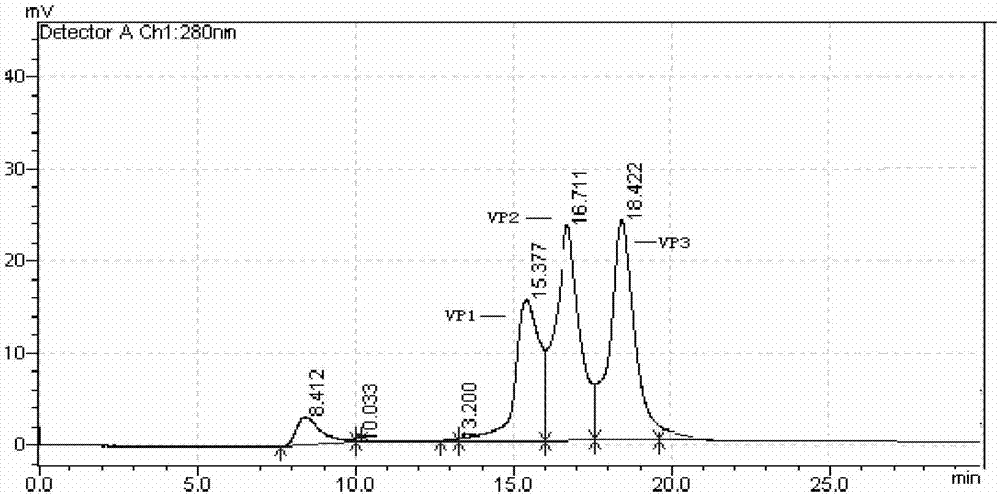
Hepatitis A virus purification method
A technology of hepatitis A virus and purification method, which is applied in the field of purification of hepatitis A virus, can solve the problems of increasing unsafe factors of hepatitis A vaccine, increasing the risk of vaccine use, and low purity of hepatitis A virus, so as to improve the quality of the virus Purity, reduced adsorption, good immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
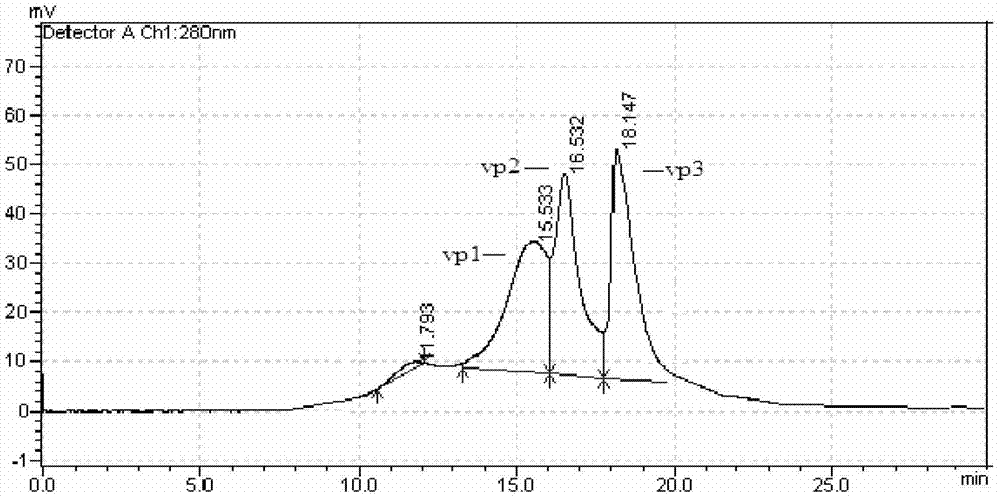
Image
Examples
Embodiment 1
[0032] The purification method of embodiment 1 hepatitis A virus and the application of preparation vaccine (1)
[0033] 1. Take 1000ml of Hepatitis A virus harvest liquid (Hepatitis A virus SH strain preservation number: CGMCC No.4501). The hepatitis A virus harvest solution (sterile antigen titer up to 1:512) was crushed in an ice bath using an ultrasonic pulverizer (purchased from Ningbo Xinzhi Company, rated power 1500w, output power 1200w) to break the cell temperature and make the cells The crushing rate is more than 99%, and the ultrasonic crushing is 4 times;
[0034] 2. Centrifuge the ultrasonically disrupted cell solution at a speed of 3500r / min for 10min, and collect the supernatant;
[0035] 3. Add chloroform to the supernatant at a volume ratio of 1:3 and shake, centrifuge at 3500r / min to absorb the upper aqueous phase; extract the protein phase and chloroform with extraction buffer (6.2mM PBS), add chloroform to shake and absorb The supernatant was extracted on...
Embodiment 2
[0054] The purification method of embodiment 2 hepatitis A virus and the application of preparation vaccine (2)
[0055] 1. Take 1000ml of Hepatitis A virus harvest liquid (Hepatitis A virus SH strain preservation number: CGMCC NO.4501). The hepatitis A virus harvest solution (sterile antigen titer up to 1:256) was crushed in an ice bath using an ultrasonic pulverizer (purchased from Ningbo Xinzhi Company, rated power 1500w, output power 1200w) to break the cell temperature and make the cells The crushing rate is above 99%, and the ultrasonic crushing is performed 5 times;
[0056] 2. Centrifuge the ultrasonically crushed cell solution at a speed of 4000r / min for 8min, and collect the supernatant;
[0057] 3. Add chloroform to the supernatant at a volume ratio of 1:2 and shake, centrifuge at 4000r / min to absorb the upper aqueous phase; then extract the protein phase and chloroform with extraction buffer (120mM NaCl), add chloroform and shake, and absorb the upper layer. Clea...
Embodiment 3
[0076] The purification method of embodiment 3 hepatitis A virus and the application of preparation vaccine (3)
[0077] 1. Take 1000ml of Hepatitis A virus harvest liquid (Hepatitis A virus strain preservation number: CGMCC NO.4501). The hepatitis A virus harvest solution (sterile antigen titer up to 1:1024) was crushed in an ice bath using an ultrasonic pulverizer (purchased from Ningbo Xinzhi Company, rated power 1500w, output power 1200w) to break the cell temperature and make the cells The crushing rate is above 99%, and the ultrasonic crushing is performed 5 times;
[0078] 2. Centrifuge the sonicated cell solution at a speed of 4500r / min for 5 minutes, and collect the supernatant;
[0079] 3. Add chloroform to the supernatant at a volume ratio of 1:1 and shake, centrifuge at 4500r / min to absorb the upper aqueous phase; then extract the protein phase and chloroform with extraction buffer (120mM NaCl), add chloroform and shake, and absorb the upper layer. Clear is 1 ext...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com