Methods to enhance wound healing and enhanced wound coverage material
a wound and material technology, applied in the field of trauma medicine and wounds, can solve the problems of high cost of materials, poor extended outcome, poor safety, etc., and achieve the effects of improving wound healing, enhancing wound healing, and enhancing wound coverag
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Experimental Animals--Rats
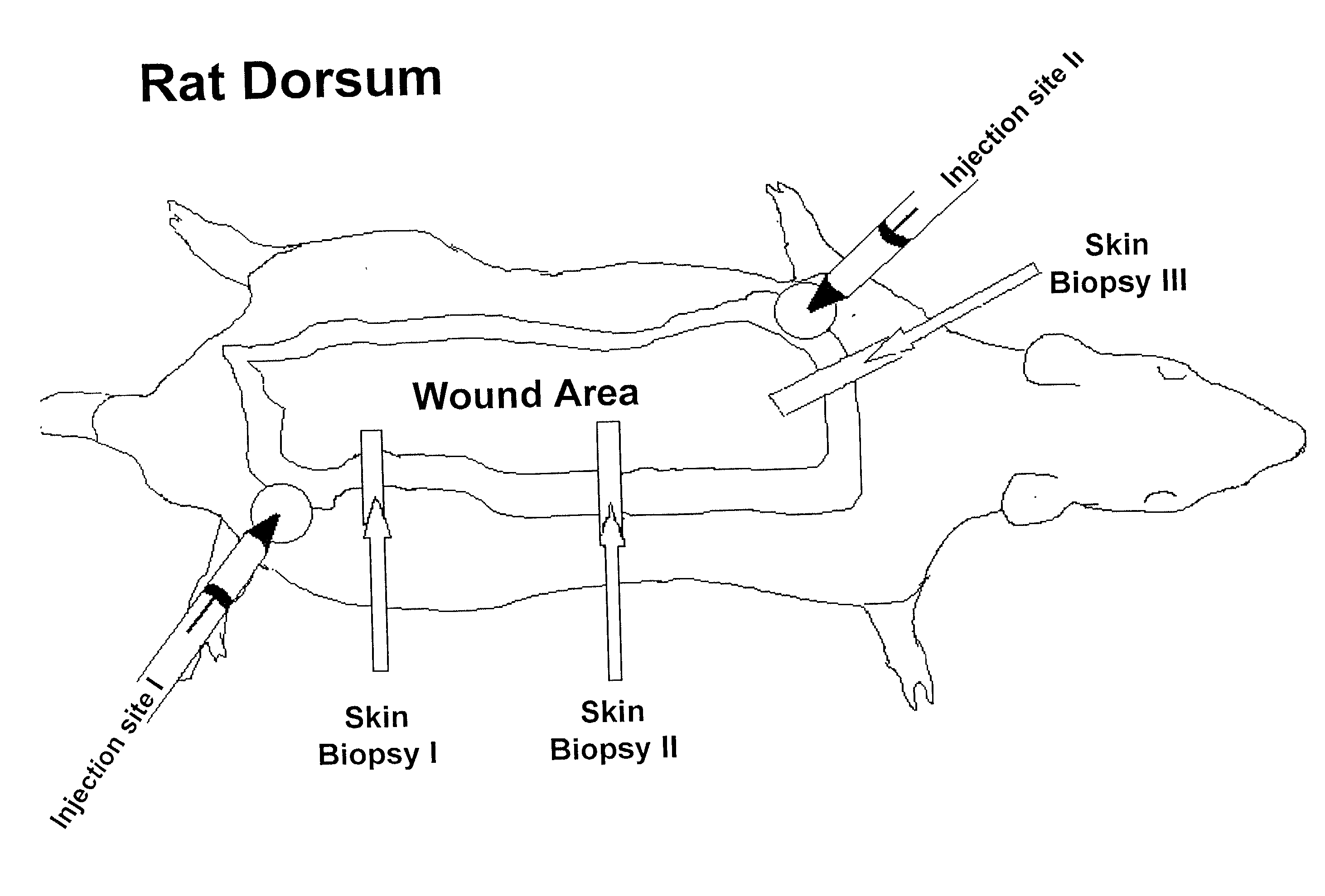
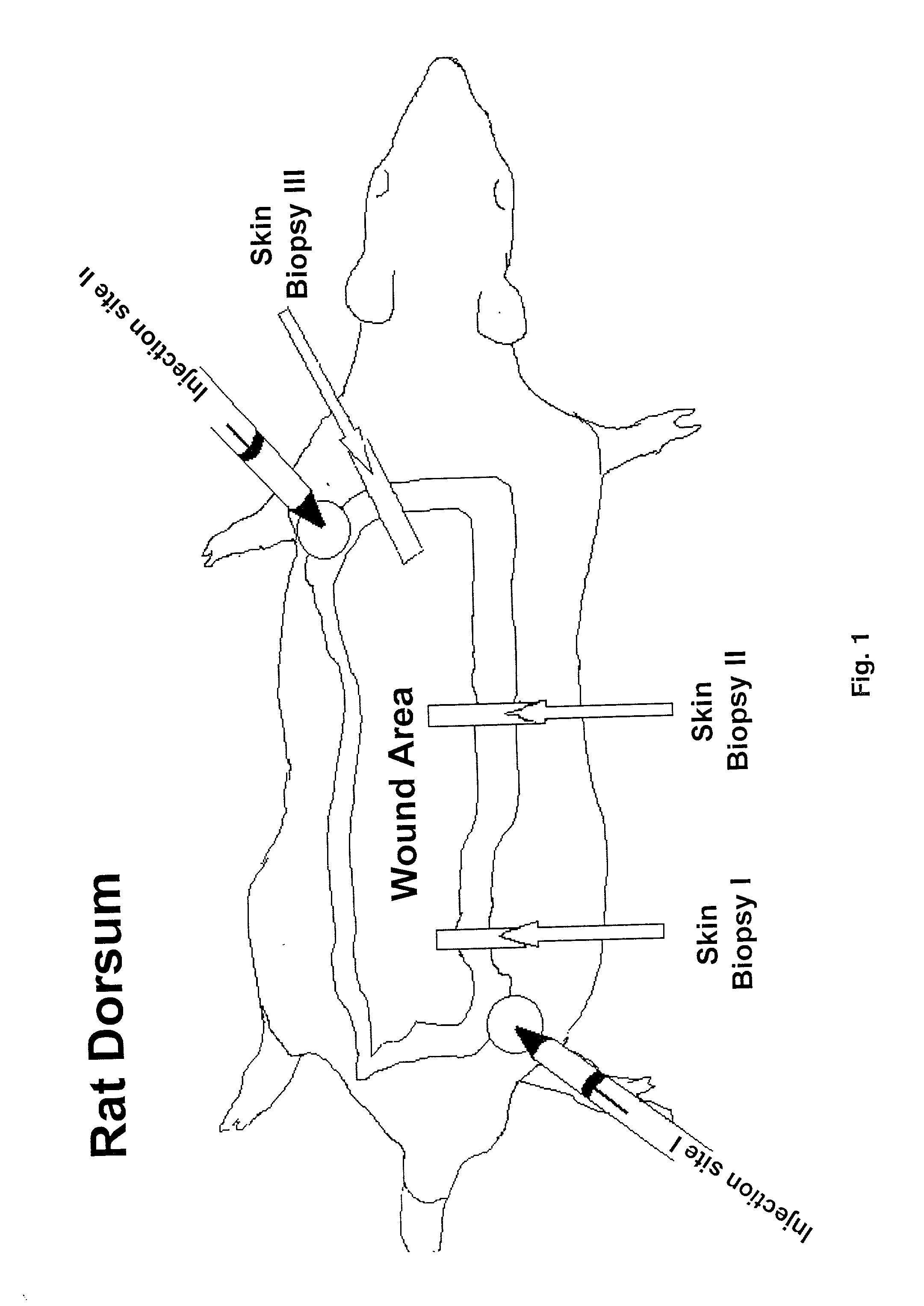
[0066] Adult male Sprague-Dawley rats (350-375 g) were placed in wire bottom cages and housed in a temperature-controlled room with a 12 hour light-dark cycle. The animals were acclimatized to their environment for 7 days prior to the start of the blinded study. All received equal amounts of a liquid diet of Sustacal (Mead Johnson Nutritionals, Evansville, Ind., USA) and water ad libitum throughout the study. Each rat received a 60% total body surface area (TBSA) full-thickness scald burn. Thermally injured rats were then randomly divided into:
[0067] (a) 2 groups to receive injections of cholesterol-containing cationic liposomes (20 .mu.l liposomes in 180 .mu.l saline, n=28), or saline (control, 200 .mu.l, n=28);
[0068] (b) 2 groups to receive weekly subcutaneous injections of liposomes (10 .mu.l liposomes in 180 .mu.l saline) containing 2.2 .mu.g of an IGF-I cDNA construct and 0.2 .mu.g of the reporter gene .beta.-galactosidase, Lac Z cDNA construct driven ...
example 2
Experimental Animals--Pigs
[0070] The wound repair mechanisms in Yorkshire mini-pigs are closest to the same mechanisms in humans. Thus, Yorkshire mini-pigs are a well-described model and have been used in several experimental trauma studies. Each of 10 Yorkshire swines receive 4 full-thickness wounds (standard model) under anesthesia and analgesia. Each wound site is square or rectangular in shape, with an approximate dimension of 8.times.8 cm, spaced approximately 5 cm between sites and confined to the area of the upper flank and back. The wounds must be positioned and placed according to a particular scheme such that the animal can lie comfortably on its side following release from the restraining hammock.
[0071] Immediately after wound induction, the wound is covered with human fetal chorion / amnion, INTEGRA.TM. (Life Science), ALLODERM.TM. (Life-Cell) or BIOBRANE.TM. (Dow-Hickhan). The cover is stapled to the unburned wound and covered with triple-antibiotic ointment-impregnated g...
example 3
Liposomes
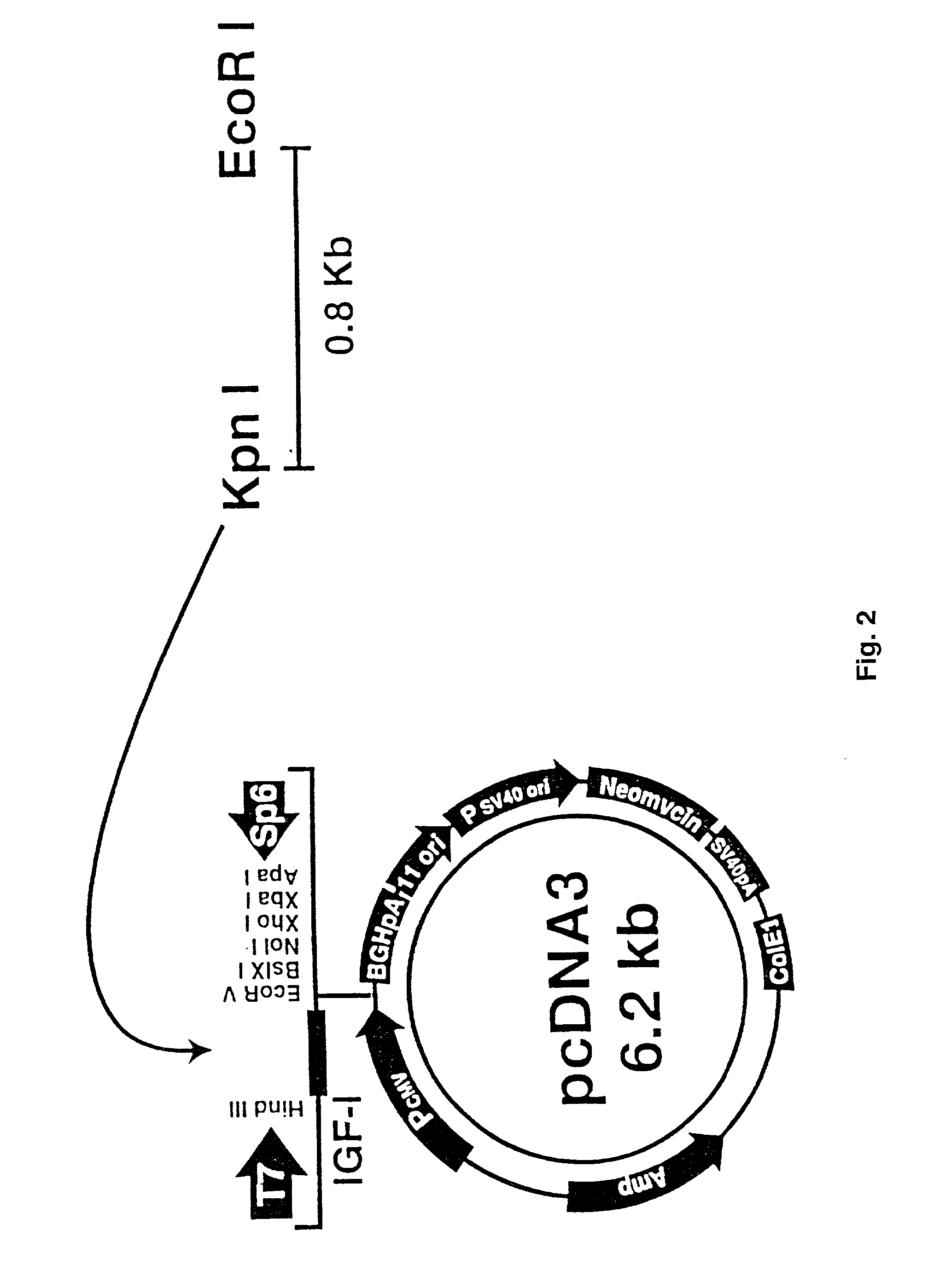
[0073] The liposomes used were cholesterol-containing cationic liposomes, DMRIE-C Reagent (1,2-dimyristyloxypropyl-3-dimethyl-hydroxyl ethyl ammonium bromide) prepared with cholesterol membrane-filtered water (Life Technologies, Rockville, Md.). The IGF-I cDNA construct (FIG. 2) consisted of a cytomegalovirus driven IGF-I cDNA plasmid prepared at the UTMB Sealy Center for Molecular Science Recombinant DNA Core Facility (the IGF-I cDNA was a kind gift of G. Rotwein, NIH, Bethesda, Md.). The doses used were 10% liposomes (the highest concentration used in DNA transfer experiments that does not have deleterious consequences on DNA solubility and is compatible with gene transfer paradigms). In order to determine the time course of liposomal effects, liposomes were injected intravenously into the tail vein of rats in group (a) 0.5 h after the thermal injury and changes were examined over a 7-day period. The volume of 200 .mu.l is an amount that can be administered into the tail ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com