HIG2 and URLC10 epitope peptide and vaccines containing the same
一种氨基酸、抗原的技术,应用在治疗和预防肿瘤的药物领域,能够解决低客观应答率等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0221] Materials and methods
[0222] cell line
[0223] PSCCA0922 (HLA-A0206) was purchased from Pharma SNP Consortium; PSC. Human B-lymphoblastoid cell line and COS7 were purchased from ATCC.
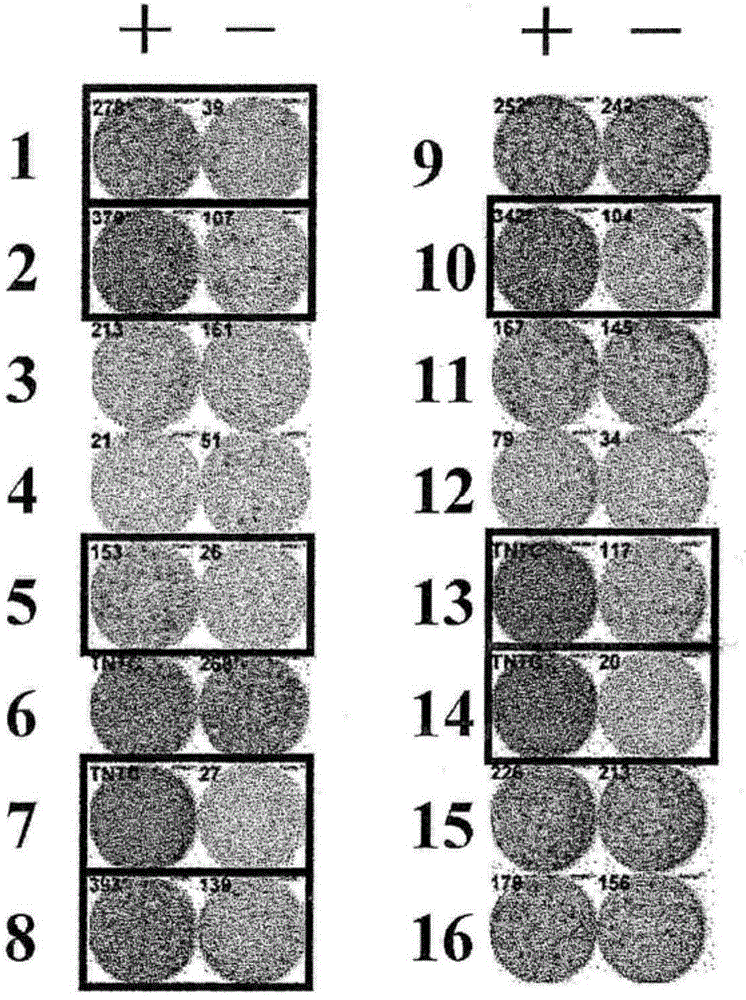
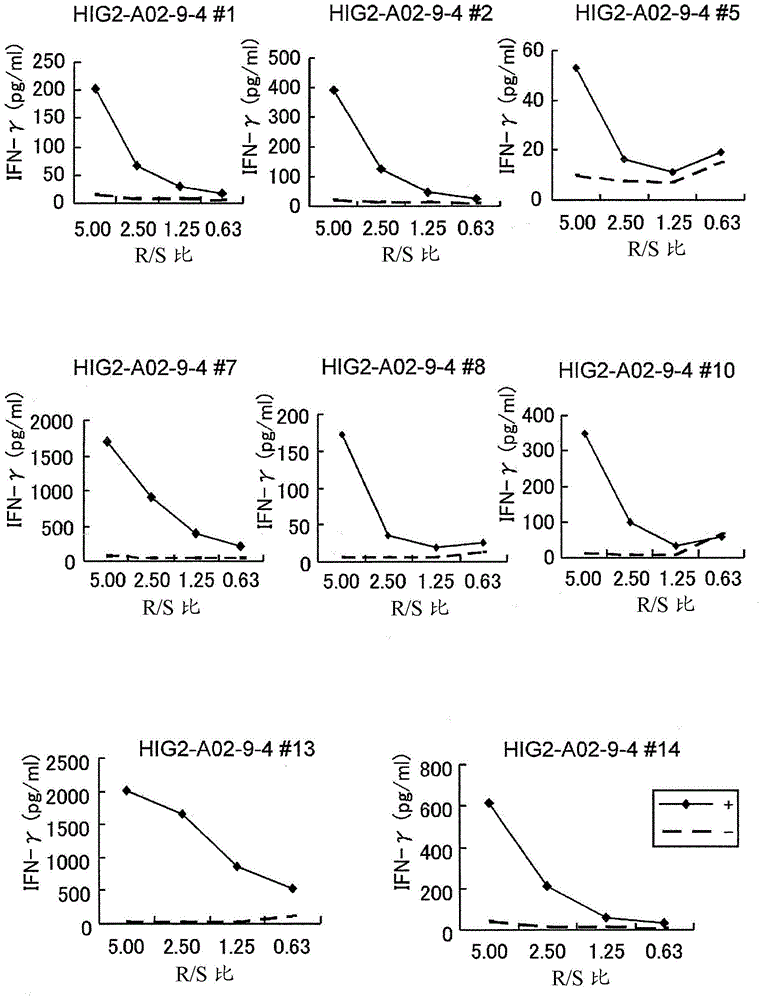
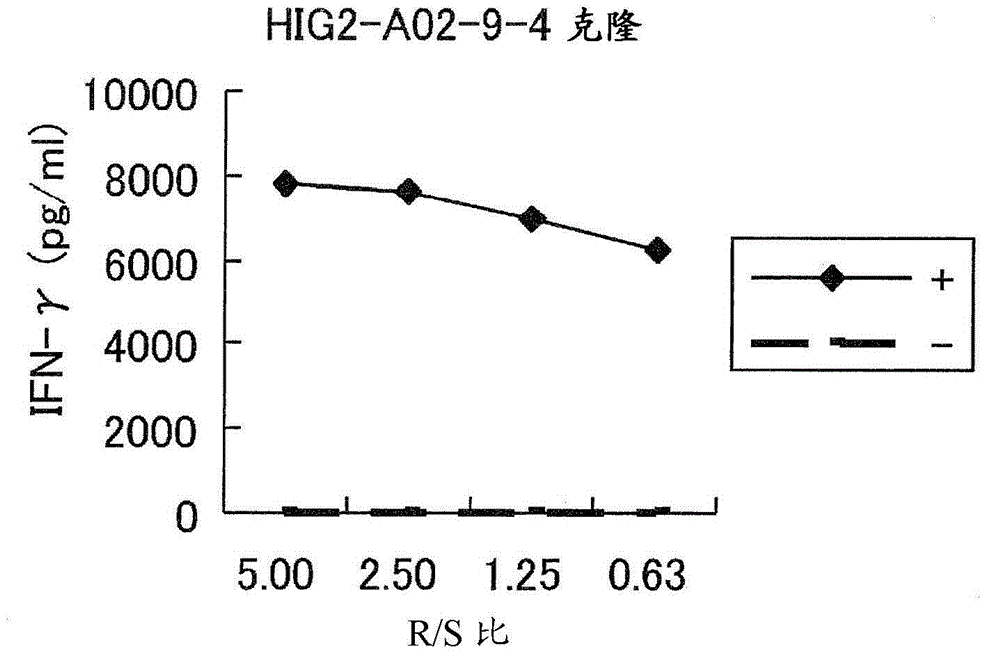
[0224] Candidate peptides derived from HIG2 or URLC10
[0225] 9-mer and 10-mer peptides derived from HIG2 or URLC10 were synthesized by Sigma (Sapporo, Japan) or Biosynthesis Inc. (Lewisville, TX) following standard solid-phase synthesis and analyzed by reverse-phase high-performance liquid chromatography (HPLC). Purification was performed. The purity (>90%) and identity of the peptides were determined by analytical HPLC and mass spectrometry analysis, respectively. Peptides were dissolved in dimethyl sulfoxide (DMSO) at 20 mg / ml and stored at -80°C.
[0226] In vitro CTL induction
[0227]Monocyte-derived dendritic cells (DCs) were used as antigen presenting cells (APCs) to induce cytotoxic T lymphocyte (CTL) responses against peptides presented on human leukocyte antige...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com