Serum amyloid protein A (SAA) antigen epitope and predication and verification method thereof as well as preparation method of monoclonal antibody
A monoclonal antibody and serum amyloid technology, applied in the field of serum amyloid A antigen epitope, raw materials for the preparation of clinical diagnostic kits in the in vitro diagnostic industry, and the preparation of monoclonal antibodies, can solve the problem of unknown recognition epitopes and lack of antibody applications , Poor antigenicity and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0089] The following will further illustrate the present invention through specific examples, but it is not intended to limit the protection scope of the present invention. Those skilled in the art can make improvements to the preparation method and the equipment used within the scope of the claims, and these improvements should also be considered as the protection scope of the present invention. Therefore, the protection scope of the patent for the present invention should be based on the appended claims.
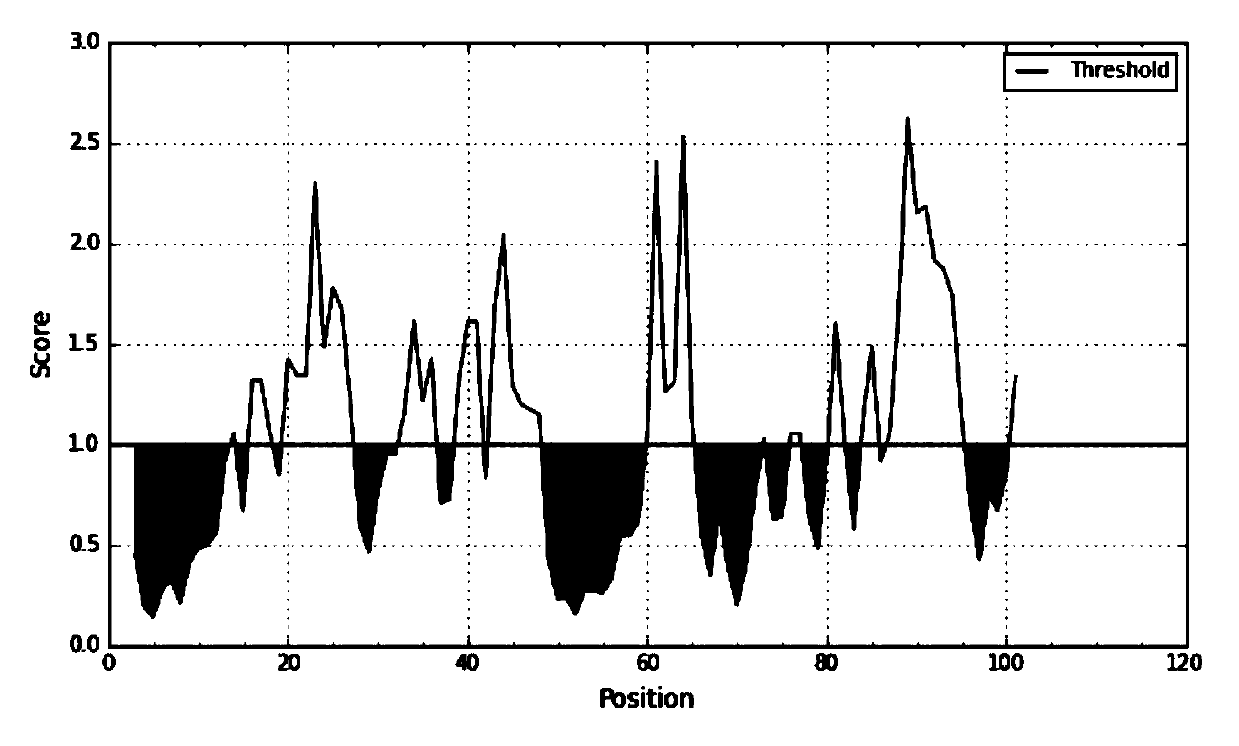
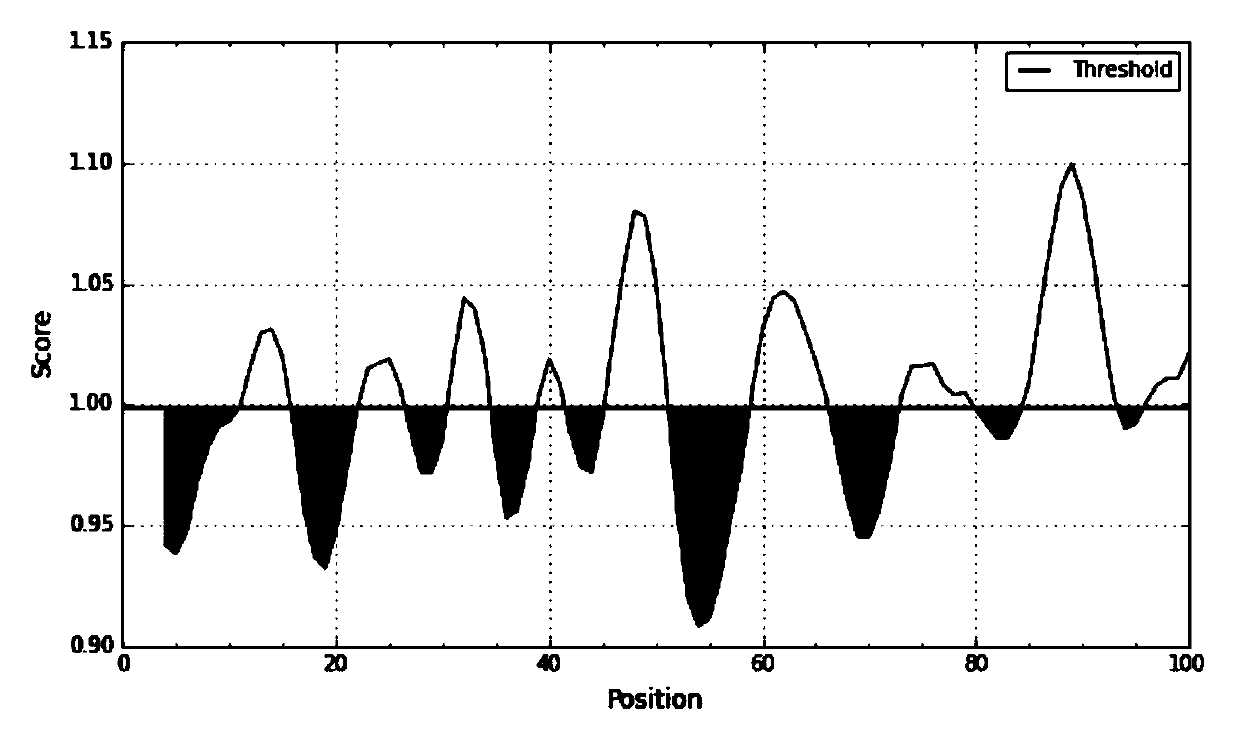
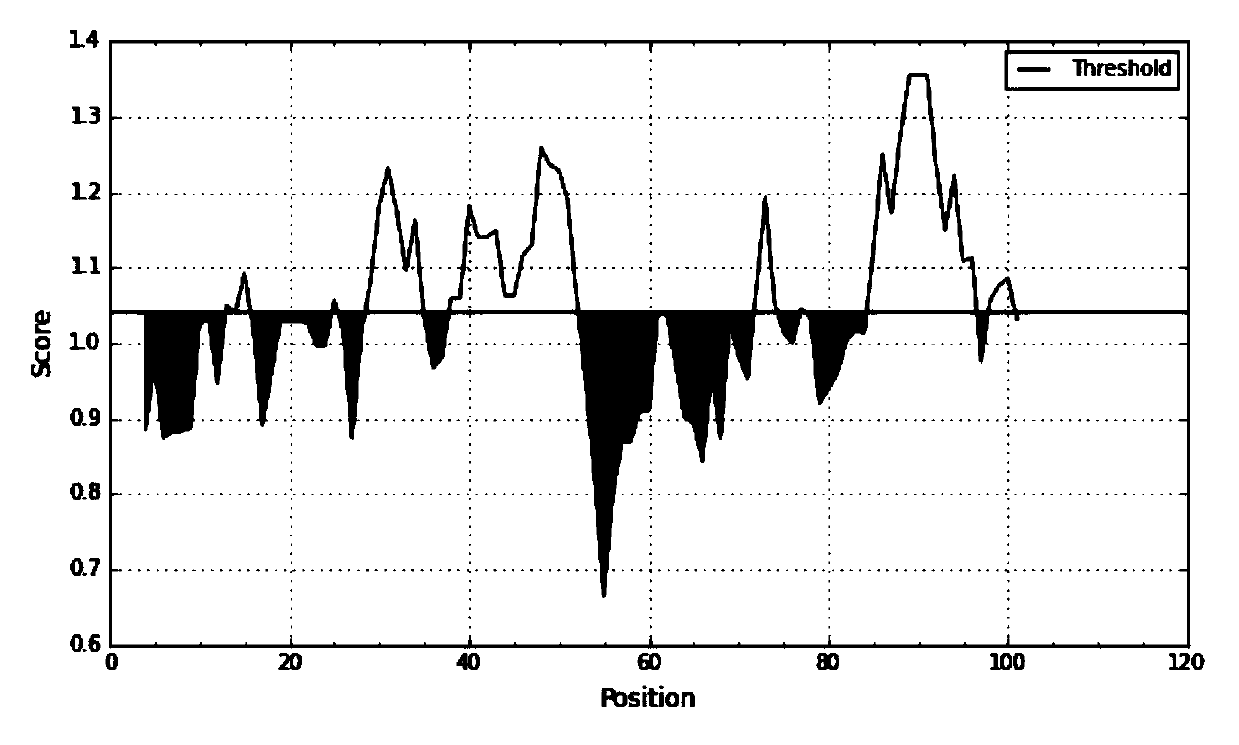
[0090] 1. SAA epitope prediction
[0091] 1. SAA sequence information was obtained through the UniProtKB database.
[0092] Protein sequence:
[0093] RSFFSFLGEAFDGARDMWRAYSDMREANYIGSDKYFHARGNYDAAKRGPGGVWAAEAIS DARENIQRFFGHGAEDSLADQAANEWGRSGKDPNHFRPAGLPEKY (SEQ ID NO. 5).
[0094] (Remarks and reference information: The full length of the SAA precursor protein is 122 amino acids, and the 18 amino acids in the N segment are signal peptides. The protein is excised during ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com