Monoclonal antibody for resisting foot and mouth disease virus type A and application thereof
A monoclonal antibody, foot-and-mouth disease virus technology, applied in the direction of anti-viral immunoglobulin, immunoglobulin, instruments, etc., can solve the problems of biological safety risks, complicated ELISA kit operation steps, unstable test results and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The acquisition of embodiment 1 hybridoma cell
[0032] 1 mouse immunization
[0033] 5-6 weeks old Balb / c mice were immunized with foot-and-mouth disease type A virus-like granule protein as the immunogen, and the immunization method was multi-point subcutaneous injection on the back, and the injection volume of each mouse was 150 μg. Freund's complete adjuvant was used for the first immunization, and Freund's incomplete adjuvant was used for subsequent booster immunizations. The time interval between each immunization was two weeks, and the mouse serum was collected one week after the third immunization to detect the antibody titer (Lan animal research liquid phase resistance ELISA kit), when the antibody titer was not lower than 1:1440, shock immunization was performed, that is, the antigen was injected into the tail vein, and three days after the shock immunization, the spleen of the mouse was taken for fusion with myeloma cells.
[0034] 2 cell fusion
[0035] Ob...
Embodiment 2
[0038] Preparation and identification of embodiment 2 monoclonal antibody
[0039] 1 Preparation of monoclonal antibody ascites
[0040] Sensitize 7-8 weeks old Balb / c mice with sterilized liquid paraffin, 0.5ml / mouse, inject 0.5ml (about 2.0×10 6 cells / ml) of hybridoma cells. After the injection, the abdomen of the mice was rubbed lightly every day, and the ascites was extracted when the abdomen of the mice was obviously enlarged laterally. Centrifuge the ascitic fluid at 10,000 rpm for 10 min, take the supernatant, aliquot and store at -70°C.
[0041] 2 Ascites potency determination of hybridoma cells
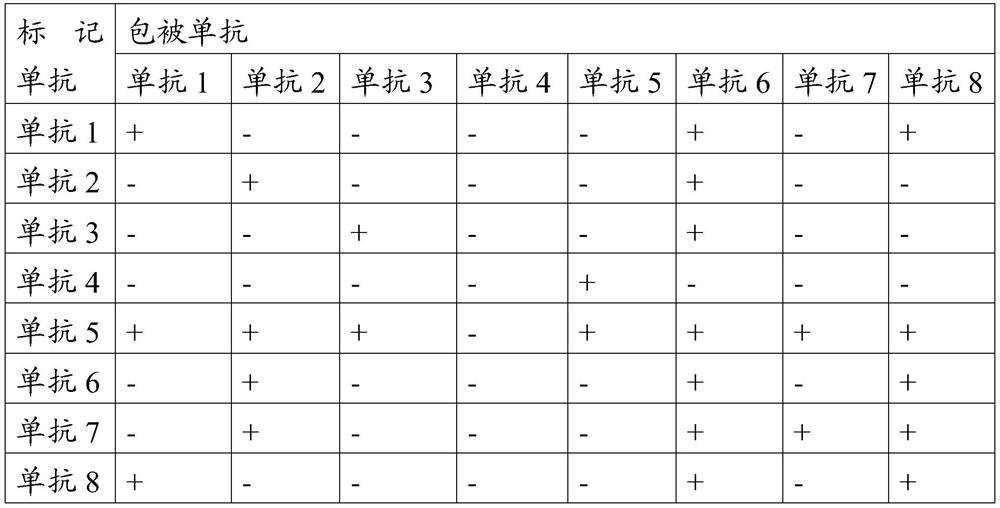
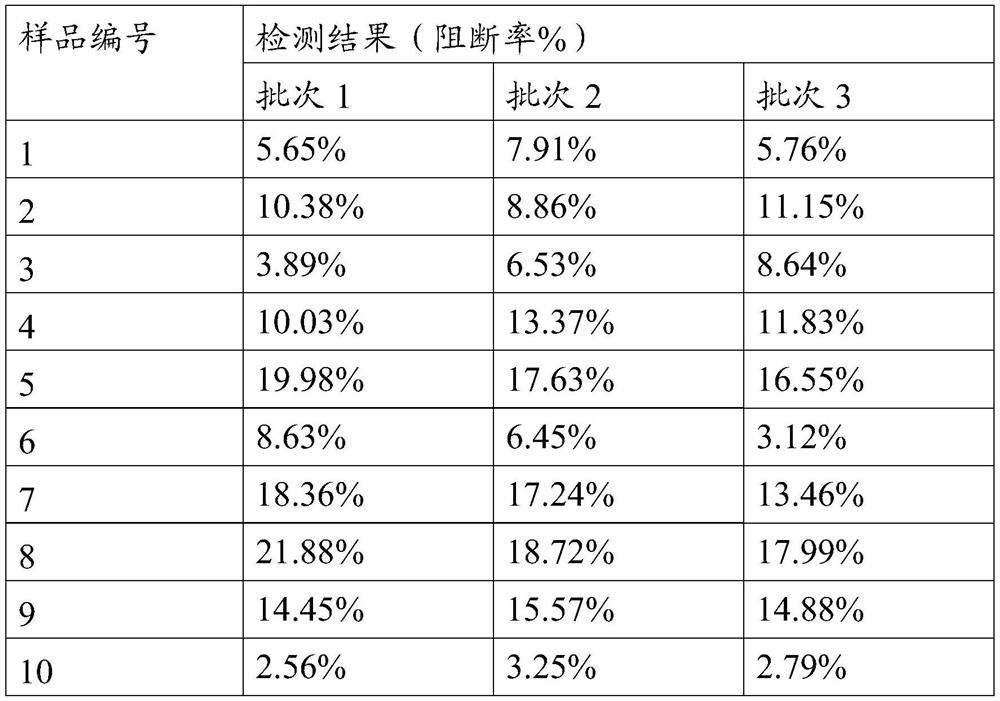
[0042] According to the instructions of the Lanshouyan Foot-and-Mouth Disease Virus Type A Antibody Liquid Phase Blocking ELISA Detection Kit, the antibody titers of the prepared monoclonal antibodies 1 to 8 were measured respectively, which were 1:5760, 1:5760, 1:8192, and 1: 2880, 1:4096, 1:5760, 1:4096, and 1:11520. It is proved that the monoclonal antibodies 1-8 have...
Embodiment 3
[0043] Purification and content determination of embodiment 3 monoclonal antibody
[0044] Use affinity chromatography to purify hybridoma mouse ascites, the specific operation is as follows: thaw the frozen ascites sample, centrifuge at 10000rpm 4°C for 10min, absorb the clear liquid, and use 3 times the column volume of sample buffer (Bindingbuffer formula : 20mM Na 2 HPO 4 , 0.15M NaCl, pH8.0) after dilution, carry out Protein G affinity chromatography column purification, column chromatography eluent is pH 2.5 0.1M glycine buffer, the eluted antibody needs to be immediately neutralized with buffer (1MTris-HCl, pH8.5) adjusted the pH value to neutral, and carried out SDS-PAGE gel analysis and protein content determination, the results showed that the purity of purified monoclonal antibodies 1 to 8 was greater than 90%, and the protein content was respectively 3.3mg / ml, 3.5mg / ml, 3.6mg / ml, 3.2mg / ml, 3.3mg / ml, 3.5mg / ml, 3.3mg / ml and 3.9mg / ml can meet the needs of clinical a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com