Human oophoroma tumor marker HE4 enzymoimmunoassay kit
An ovarian cancer and detection kit technology, applied in microorganisms, plant genetic improvement, botanical equipment and methods, etc., can solve problems such as limiting clinical application, and achieve the effects of simple operation, high cost performance and high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Example 1: Construction, protein expression, purification and identification of HE4 gene
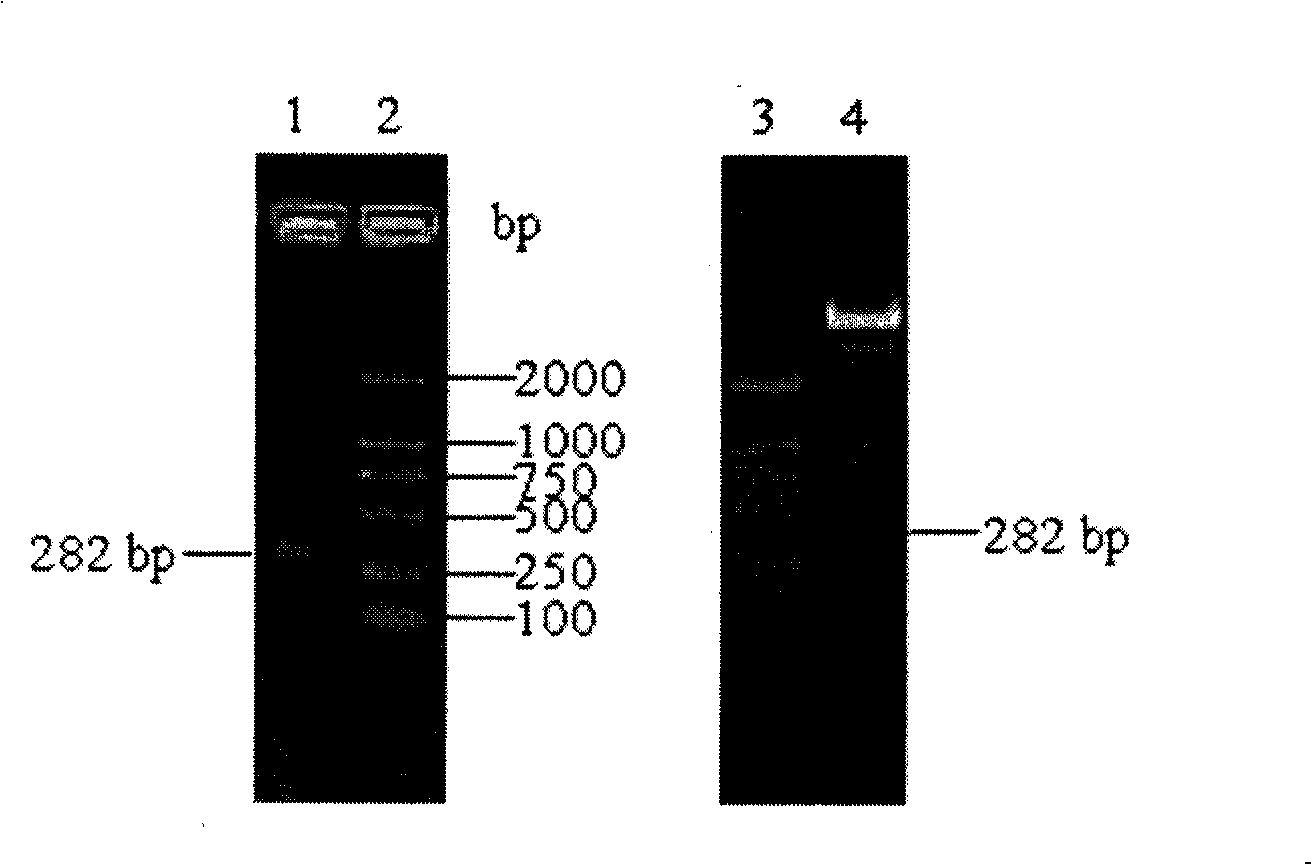
[0015] 1. Amplification of HE4 gene
[0016] According to the human HE4 mRNA sequence (NM_006103) provided in Genbank, 30 signal peptides at the N-terminus were removed, and 8 primers were designed and synthesized according to the preferred codons of Escherichia coli, respectively named P1-P8. The primers were synthesized by Dalian Bao Biological Company.
[0017] P1~P8 nucleotide sequence composition:
[0018] P1: tggttgtgctactttttgttctttacctaatgataaagaaggttcttgtc
[0019] P2: ctaattgaggaaaattaatattaacttgaggacaagaaccttctttatca
[0020] P3: tctgaatgtgctgataatttaaaatgttgttctgctggttgtgctacttt
[0021] P4: ttgagaatcaacttgacattgatcacgacataaacctaattgaggaaaat
[0022] P5: ctgatcaaaattgtactcaagaatgtgtttctgattctgaatgtgctgat
[0023] P6: caaccattacgacaacatttcatttgaccaggacattgagaatcaacttg
[0024] P7: cgggatccgaaaaaactggtgtttgtcctgaattacaagctgatcaaaattgta
[0025] P8: ccgctcgagtttatt...
Embodiment 2
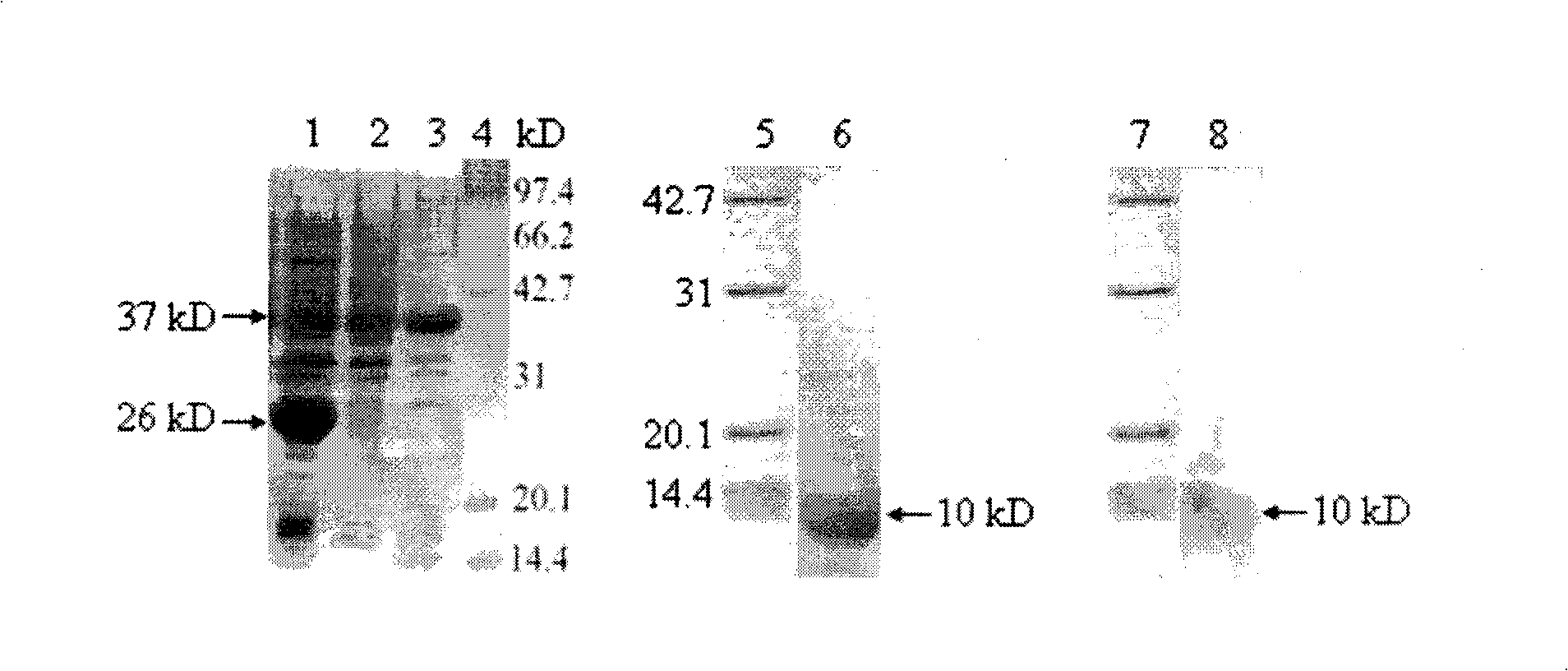
[0052] Example 2: Preparation of rabbit anti-human HE4 polyclonal antibody
[0053] 1. Animal immunization
[0054] Take 500 μl (about 500 μg) of purified HE4 protein, fully emulsify it with an equal amount of Freund’s complete adjuvant, and immunize New Zealand big-eared white rabbits by subcutaneous multi-point immunization method on the back, and then immunize once every two weeks. The protein immunization dose is 300 μg, The adjuvant was Freund's incomplete adjuvant, and the whole process was immunized for 4 times, and blood was collected from the ear vein of rabbits seven days after the last immunization. After the antibody titer was determined by indirect ELISA, the carotid artery was bled, and the serum was collected and stored at -20°C.
[0055] 2. Purification and potency identification of rabbit anti-human HE4 polyclonal antibody
[0056] Rabbit serum was purified by Protein G affinity chromatography, and the serum was diluted with binding buffer (20mM sodium phosp...
Embodiment 3
[0059] Example 3: Preparation of mouse anti-human HE4 monoclonal antibody
[0060] 1. Animal immunization
[0061] Take 300 μl (about 300 μg) of purified HE4 protein and fully emulsify it with an equal amount of complete Freund’s adjuvant, inject BALB / c mice subcutaneously in multiple points on the back, and immunize 3 mice in total. One week later, each mouse was subcutaneously injected with 50 μg antigen dose and the same amount of Freund’s incomplete adjuvant fully emulsified, and then immunized once every two weeks. For intraperitoneal injection, from the second immunization, the mouse blood was collected from the tail vein on the seventh day after each immunization, and the serum titer was detected by indirect ELISA (the coated antigen was purified HE4 protein), and the titer reached 1:50000 After the above preparations for fusion, 3 days before fusion, a booster immunization was injected into the tail vein once, and the antigen dose was 50 μg.
[0062] 2. Establishment...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com