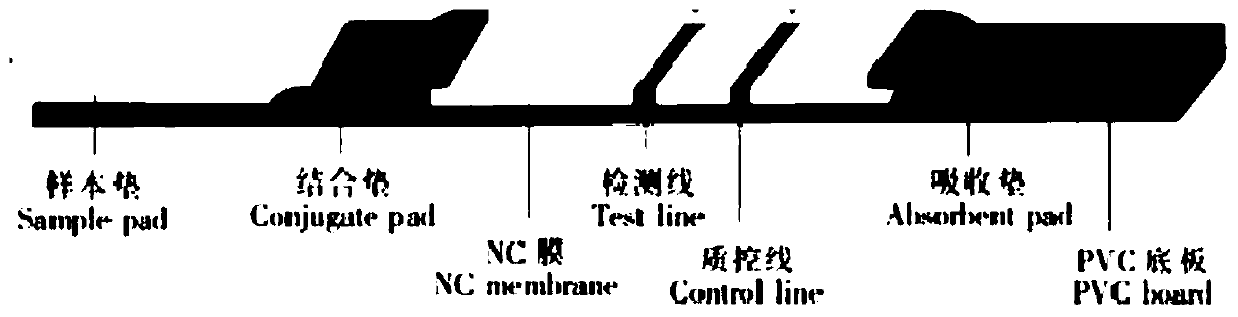
Detection kit of IgM/IgG antibodies of novel coronavirus (SARS-CoV-2)
A sars-cov-2 and detection test paper technology, applied in the direction of virus/bacteriophage, virus, virus peptide, etc., can solve the problems that the detection results are greatly affected by the RNA extraction effect, quality and aerosol pollution, and cannot meet the detection requirements, etc. Achieve short detection time, improve reaction sensitivity, and reduce missed detection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0096] In a preferred embodiment, the preparation method of the binding pad gold-labeled protein comprises: mixing and reacting the colloidal gold solution with the mouse anti-label antibody to obtain the mouse anti-label antibody-colloidal gold cross-linked complex, and then mixing and reacting with nCOVAg01, Obtain labeled antigen 1; mix and react the colloidal gold solution with rabbit IgG to obtain labeled antibody 2; mix labeled antigen 1 and labeled antigen 2 at a molar ratio of 2-4:7 to obtain gold-labeled protein.
[0097] In a more preferred embodiment, nCOVAg01 is a protein composed of the amino acid sequence described in SEQ ID NO.9, and the mouse anti-Trx antibody is first labeled with colloidal gold to obtain a mouse anti-Trx antibody-colloidal gold complex, and then nCoVAg01 is labeled with mouse On the anti-Trx antibody-colloidal gold complex, the labeled antigen 1 is obtained; the colloidal gold solution is mixed with rabbit IgG to obtain the labeled antibody 2;...
Embodiment 1
[0101] Example 1 Antigen Sequence Design
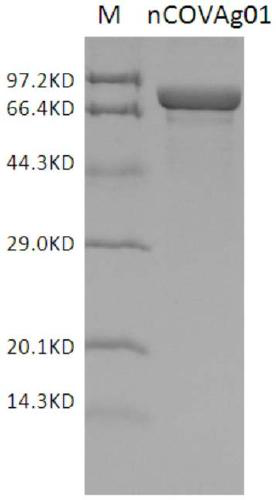
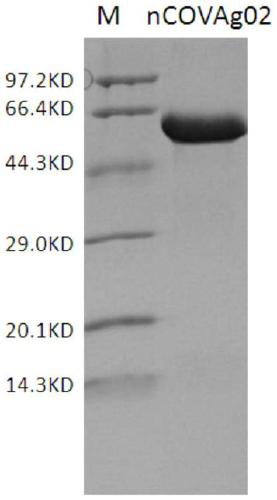
[0102]According to the known complete gene sequence of SARS-CoV-2 coronavirus NC_045512.2, the recombinant expression antigens, nCoVAg01 and nCoVAg02, were designed. Among them, the antigenic structure of nCOVAg01 is Trx+N+S+hydrophilic sequence+His, and the antigenic structure of nCOVAg02 is His+N+S+His. Specifically, the nCOVAg01 antigen is a protein composed of the amino acid sequence described in SEQ ID NO.9; nCOVAg02 is a protein composed of the amino acid sequence described in SEQ ID NO.10.
Embodiment 2
[0103] Embodiment 2 antigen construction
[0104] Three sequences of whole gene synthesis N01 (SEQ ID NO.11), S01 (SEQ ID NO.12) and S02 (SEQ ID NO.13) were used to construct the antigen.
[0105] N01-pUC plasmid was digested with NcoI and NheI to obtain fragment 1, S01-pUC plasmid was digested with NheI and XhoI to fragment 2, and fragment 1 and fragment 2 were connected to pET30a-Trx vector backbone (NcoI and XhoI) to transform Escherichia coli DH5α, positive clones were screened, and the nCoVAg01 plasmid was identified by sequencing.
[0106] The N01-pUC plasmid was digested with NdeI and NheI to obtain fragment 3, the S02-pUC plasmid was digested with NheI and XhoI to obtain fragment 4, and fragment 3 and fragment 4 were connected to the pET28a vector backbone (NdeI and XhoI) to transform Escherichia coli DH5α, Positive clones were screened, and the nCoVAg02 plasmid was identified by sequencing.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com