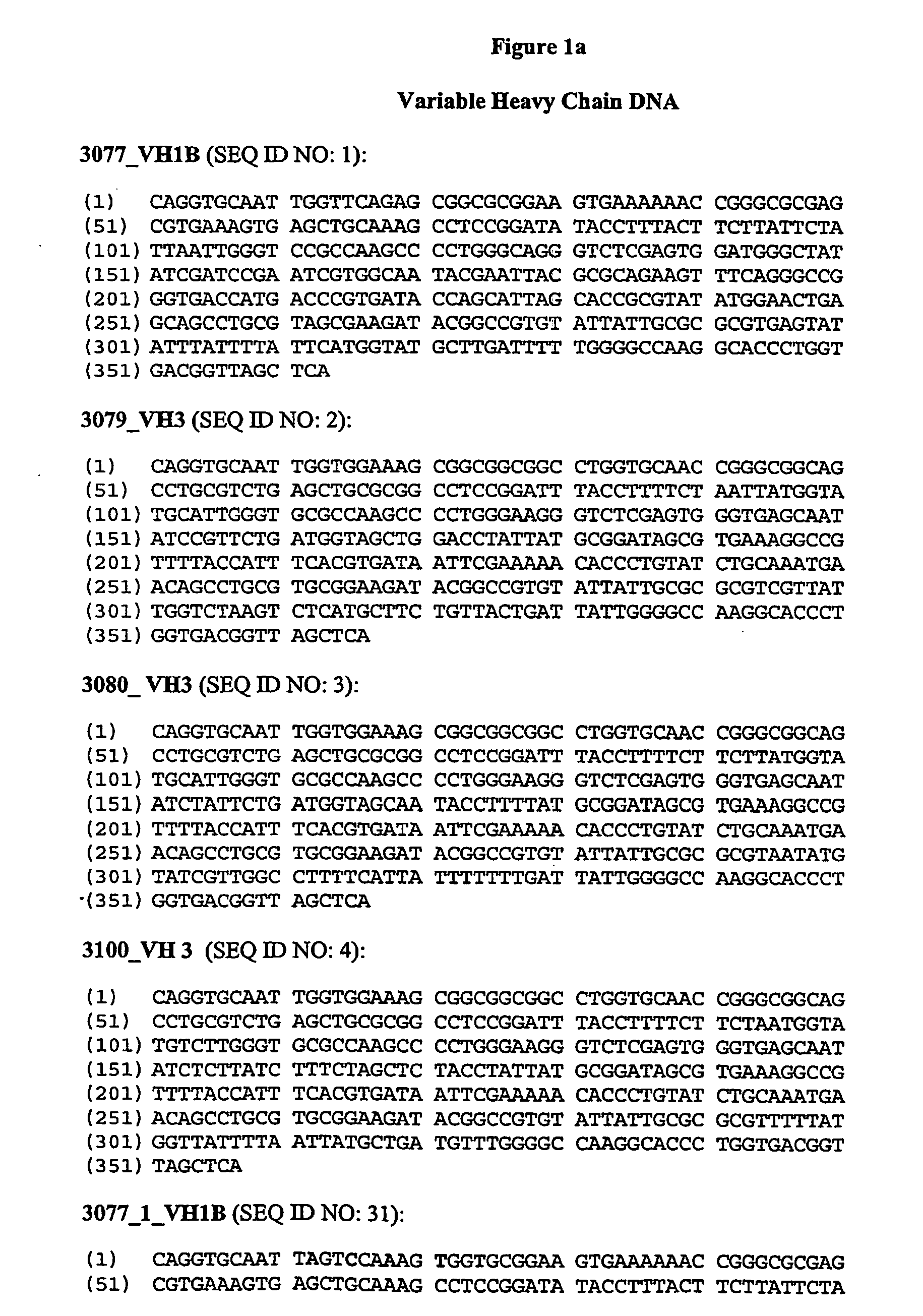
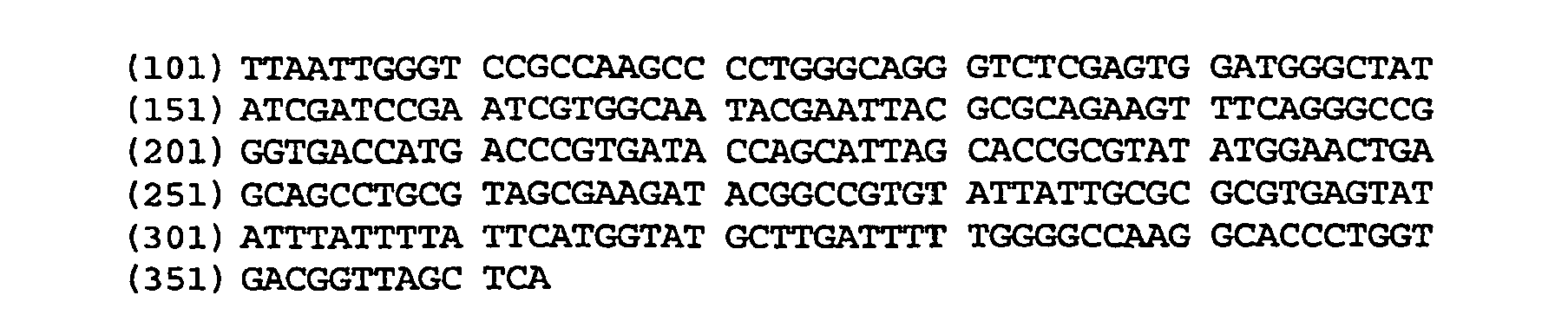Anti-CD38 human antibodies and uses thereof
a human antibody and anti-cd38 technology, applied in the field of anti-cd38 human antibodies, can solve the problems of inconvenient human administration, low affinity and efficacy of okt10, and achieve the effect of effectively mediating the killing of cd38-overexpressing cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Antibody Generation from HuCAL Libraries
[0123]For the generation of therapeutic antibodies against CD38, selections with the MorphoSys HuCAL GOLD phage display library were carried out. HuCAL GOLD® is a Fab library based on the HuCAL® concept (Knappik et al., 2000; Krebs et al., 2001), in which all six CDRs are diversified, and which employs the CysDisplay™ technology for linking Fab fragments to the phage surface (Löbning, 2001).
A. Phagemid Rescue, Phage Amplification and Purification
[0124]HuCAL GOLD® phagemid library was amplified in 2×TY medium containing 34 μg / ml chloraunphenicol and 1% glucose (2×TY-CG). After helper phage infection (VCSM13) at an O600 of 0.5 (30 mint 37° C. without shaking; 30 min at 37° C. shaking at 250 rpm), cells were spun down (4120 g; 5 min; 4° C.), resuspended in 2×TY / 34 μg / ml chloramphenicol / 50 μg / ml kanamycin and grown overnight at 22° C. Phages were PEG-precipitated from the supernatant, resuspended in PBS / 20% glycerol and stored at −80° C. Phage amp...
example 2
Biological Assays
[0125]Antibody dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity was measured according to a published protocol based on flow-cytometry analysis (Naundorf et al., 2002) as follows:
ADCC:
[0126]For ADCC measurements, target cells (T) were adjusted to 2.0E+05 cells / ml and labeled with 100 ng / ml Calcein AM (Molecular Probes, C-3099) in RPMI164O medium (Pan biotech GmbH) for 2 minutes at room temperature. Residual calcein was removed by 3 washing steps in RPMI1640 medium. In parallel PBMC were prepared as source for (natural killer) effector cells (E), adjusted to 1.OE+07 and mixed with the labeled target cells to yield a final B:T-ratio of 50:1 or less, depending on the assay conditions. Cells were washed once and the cell-mix resuspended in 200 μl RPMI1640 medium containing the respective antibody at different dilutions. The plate was incubated for 4 hrs under standardized conditions at 37° C. and 5% CO2 in a humidified incubator. Prior to FAC...
example 3
Generation of Stable CD38-Transfectants and CD38 Fc-Fusion Proteins
[0130]In order to generate CD38 protein for panning and screening two different expression systems had to be established. The first strategy included the generation of CD38-Fc-fusion protein, which was purified from supernatants after transient transfection of HEK293 cells. The second strategy involved the generation of a stable CHO-K1-cell line for high CD38 surface expression to be used for selection of antibody-phages via whole cell panning.
As an initial step Jurkat cells (DSMZ ACC282) were used for the generation of cDNA (Invitrogen) followed by amplification of the entire CD38-coding sequence using primers complementary to the first 7 and the last 9 codons of CD38, respectively (primer MTE001 & MTE002rev; Table 4). Sequence analysis of the CD38-unsert confirmed the published amino acid sequence by Jackson et al. (1990) except for position 49 which revealed a glutamune instead of a tyrosine as described by Nata e...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com