Infectious bursal disease virus subunit antigen-containing vaccine composition, preparation method and application thereof
A vaccine composition and chicken infectivity technology, applied in the field of veterinary medicine, can solve the problems of negative reaction, high production cost, high endotoxin content, etc., and achieve the effect of reducing negative reaction, production cost and side reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1: Construction of pColdⅢ_IBDV_VP2 expression system
[0049] 1. Experimental materials
[0050] Plasmid extraction kit was purchased from Tiangen Biology; T4 DNA Ligase was purchased from BioLab; EcoR1, Sal1 restriction endonuclease, pColdⅢ_DH5α strain were purchased from TaKaRa; agarose gel recovery kit was purchased from Tianze Biology, and other reagents were Analytical pure.
[0051] 2. Experimental steps
[0052] 2.1 Preparation of VP2 cDNA
[0053] 2.1.1 Extraction of total RNA
[0054] The brief process of the total RNA is as follows: grind the bursa of SPF chickens infected with the supervirulent LQ9 strain of chicken infectious bursal disease virus with a grinder. Take 200 μL of disease material and add TE (10mM Tris, 1mM EDTA, pH8.0) to 500 μL, add 5 μL of proteinase K and 50 μL of 10% (W / V) sodium dodecylsulfonate (SDS), and bathe in water at 56°C for 3 hours . Add an equal volume of phenol / chloroform (1:1, V / V) to extract three times, and extr...
Embodiment 2
[0079] Embodiment 2: IBDV VP2 protein preparation
[0080] 1. Experimental materials: pColdⅢ-VP2 / E.Coli BL21 (DE3) strain, IPTG, PBS.
[0081] 2. Experimental method
[0082] 2.1 Prepare LB medium containing 50-100 μg / ml ampicillin.
[0083] 2.2. Inoculate the culture medium containing the pColdⅢ-VP2 / E.Coli BL21 (DE3) strain prepared in Example 1, the inoculum amount is 1% (V / V), and culture with shaking at 37°C.
[0084] 2.3. When OD600=0.4-0.6, place it at 15°C for 30 minutes.
[0085] 2.4. Add isopropyl-β-D-thiogalactopyranoside (IPTG) so that the final concentration is 0.1-1.0mM, and culture with shaking at 15°C for 24 hours.
[0086] 2.5. After the cultivation, collect the bacteria, and use PBS (sodium chloride, 8g, potassium chloride, 0.2g, disodium hydrogen phosphate, 1.44g, potassium dihydrogen phosphate, 0.24g, adjust pH 7.4, constant volume 1L) The bacteria were resuspended, ultrasonically disrupted, and centrifuged to take out the previous agarose diffusion test...
Embodiment 3
[0089] Example 3: Escherichia coli expresses VP2 protein endotoxin clearance
[0090] 1. Experimental materials:
[0091] The antigen prepared in Example 2; endotoxin detection Limulus test kit and endotoxin standard were purchased from Xiamen Limulus Reagent Company, and other reagents were of analytical grade.
[0092] 2. Experimental method
[0093] 2.1 Triton X-114 solution for removing endotoxin from crude VP2 protein
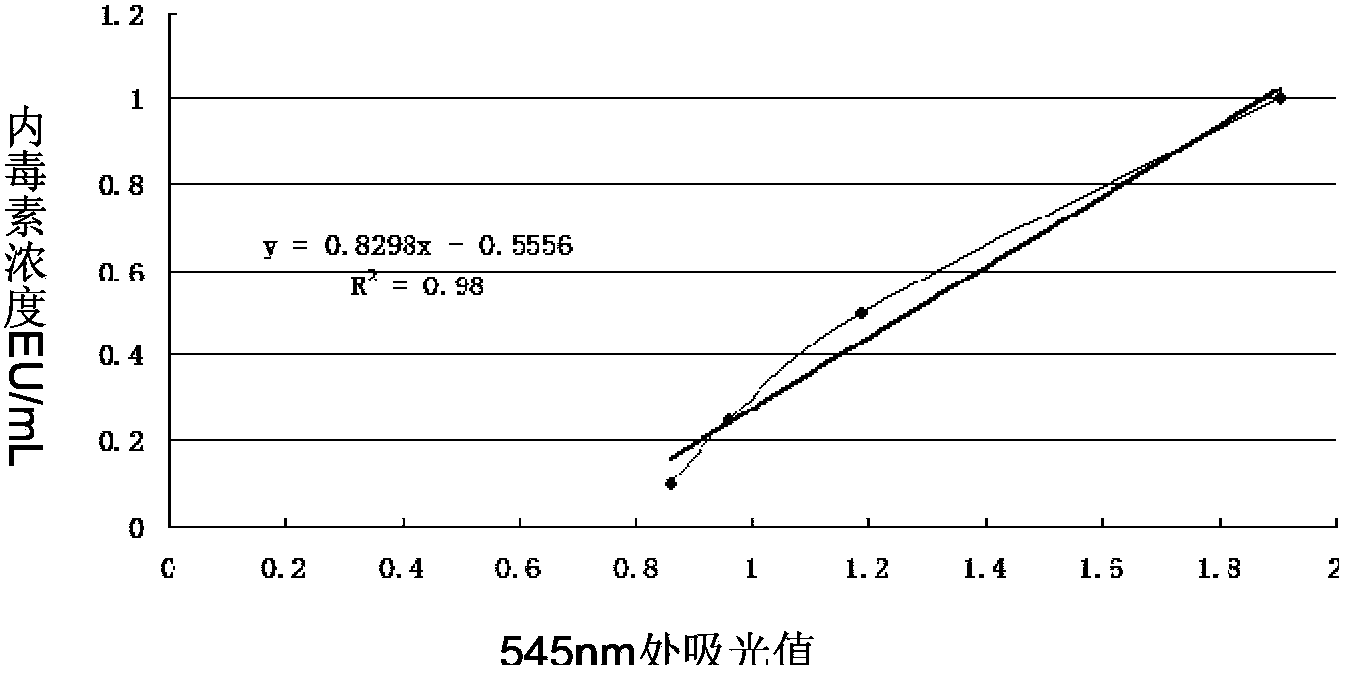
[0094] Add 0.5ml of the solution to be treated and Triton X-114 (5μl) at a final concentration of 1% (v / v) to a 1.5ml centrifuge tube, and vortex. Samples were placed on ice for 5 minutes. After vortexing the cooled samples, the centrifuge tubes were immediately placed in a 37°C water bath for 5 min to allow a new two-phase formation. Then, the samples were centrifuged at 37°C for 60 s. After centrifugation, the target protein will remain in the upper layer, while the detergent containing endotoxin will remain at the bottom of the centrifuge tube in t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com