Bacterin for pleuropneumonia actinobacillus serotype 1 double-gene deletion mutant without resistance marker
A technology of porcine pleuropneumonia and actinobacillus, applied in the direction of antibacterial drugs, bacteria, bacterial antigen components, etc., can solve the problems of not meeting the biological safety requirements, not being able to be used as vaccine strains, etc., and achieve the protection of homologous and heterologous serotypes Bacteria attack, broad market application prospects, strong virulence effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
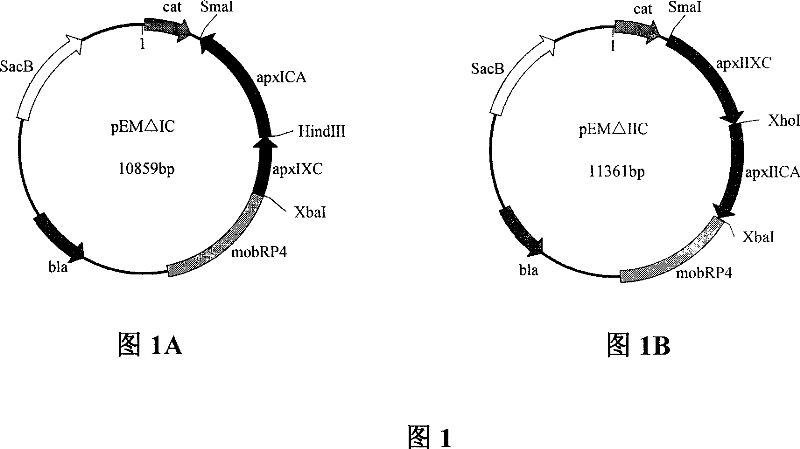
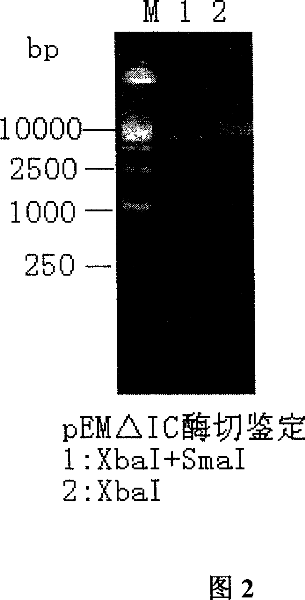
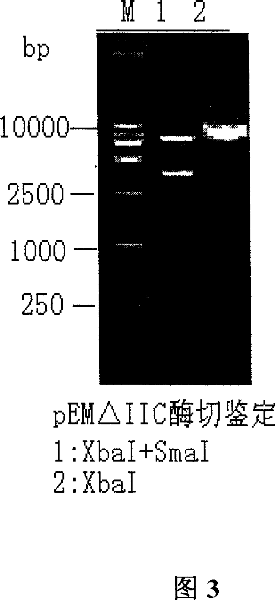
Image
Examples
Embodiment 1
[0042] 1. Primer design (for gene cloning and molecular detection)
[0043] According to the reported sequence of APP-1 strain (refer to the gene sequence of GenBank accession number X68595), four pairs of primers were designed to amplify the upstream arm and downstream arm of the toxin apxI activating gene apxIC, respectively. The size of the amplified fragments were 800bp and 1700bp, and the upstream arm Both ends are designed with EcoRI and HindIII restriction sites, and both ends of the downstream arm are designed with HindIII and XhoI restriction sites; the upstream and downstream arms of the activation gene apxIIC of apxII, the amplified fragment size is 1600bp and 1800bp, respectively, the upstream arm PstI and EcoRI restriction sites were designed at both ends, and KpnI and XhoI restriction sites were designed at both ends of the downstream arm. The above primers were all synthesized by Shanghai Bioengineering Company. The primer sequence is as follows:
[0044] pI-1: 5’-A...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com