Patents
Literature
82 results about "Gene deletion mutation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Genetic rearrangement through loss of segments of DNA or RNA, bringing sequences which are normally separated into close proximity.
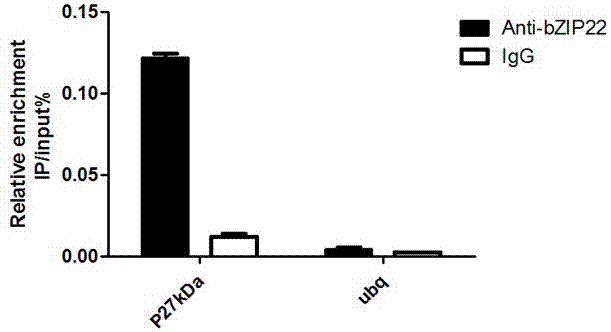
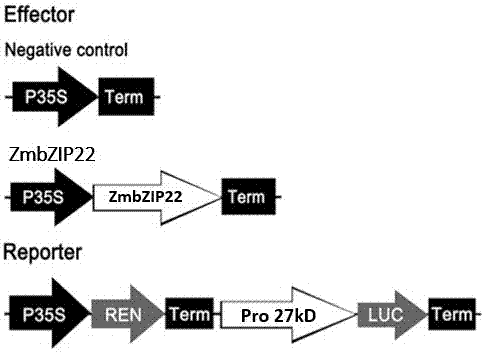
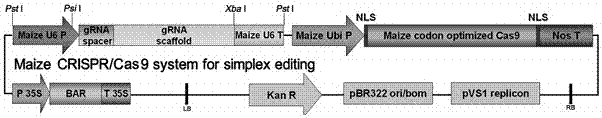
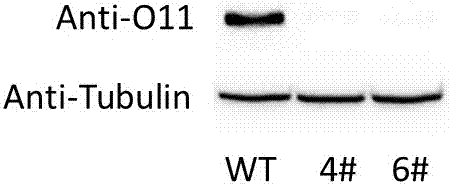
Zea mays transcription factor ZmbZIP22 and application thereof
ActiveCN107298701AReduce accumulationIncrease methionine contentPlant peptidesFermentationWild typeEssential amino acid
The invention relates to an application of a Zea mays grain transcription factor in the aspect of regulation and control of alcohal-soluble proteins. The factor comprises a base sequence shown in SEQ ID NO: 1. The protein ZmbZIP22 encoded by the sequence can be directly bonded to a 27kDa gamma-gliadin promoter and activate the 27kDa gamma-gliadin promoter. Gene-deficient mutant plants are obtained through carrying out Zea mays immature-embryo conversion by taking a gene fragment, shown in SEQ ID NO: 2, of ZmbZIP22 as a guide RNA by using a CRISPR-Cas9 technology. Compared with wild type grains, transgenic mutants have Zea mays grains with irregular and relative-thin protein body shells, the content of alcohal-soluble proteins in mature grains is remarkably lowered, the content of essential amino acids such as lysine, which are deficient to the conventional Zea mays, in the mature grains is remarkably increased, and thus, the nutritional quality of Zea mays is improved.
Owner:SHANGHAI UNIV
Corn transcription factor ZmbHLH167 and application thereof
The invention relates to a corn transcription factor ZmbHLH167 and an application of the corn transcription factor ZmbHLH167. A base sequence of the gene is shown as SEQ ID NO (sequence identifier number): 1. A corn immature embryo is transformed by taking a gene segment SEQ ID NO: 2 of the sequential coding protein ZmbHLH167 as guide RNA (ribonucleic acid) by use of a CRISPR (clustered regularly interspaced short palindromic repeats)-Cas9 technology, and a plant of a gene deletion mutant is obtained. Compared with a wild seed, a corn seed of the genetically modified mutant is smaller significantly, but a germination rate is not influenced. Biochemical analysis shows that a starch content of the corn seed of the genetically modified mutant is decreased obviously, a content of protein and total oil and fat is significantly increased, and a genetic resource is provided for creating high quality corn.
Owner:SHANGHAI UNIV
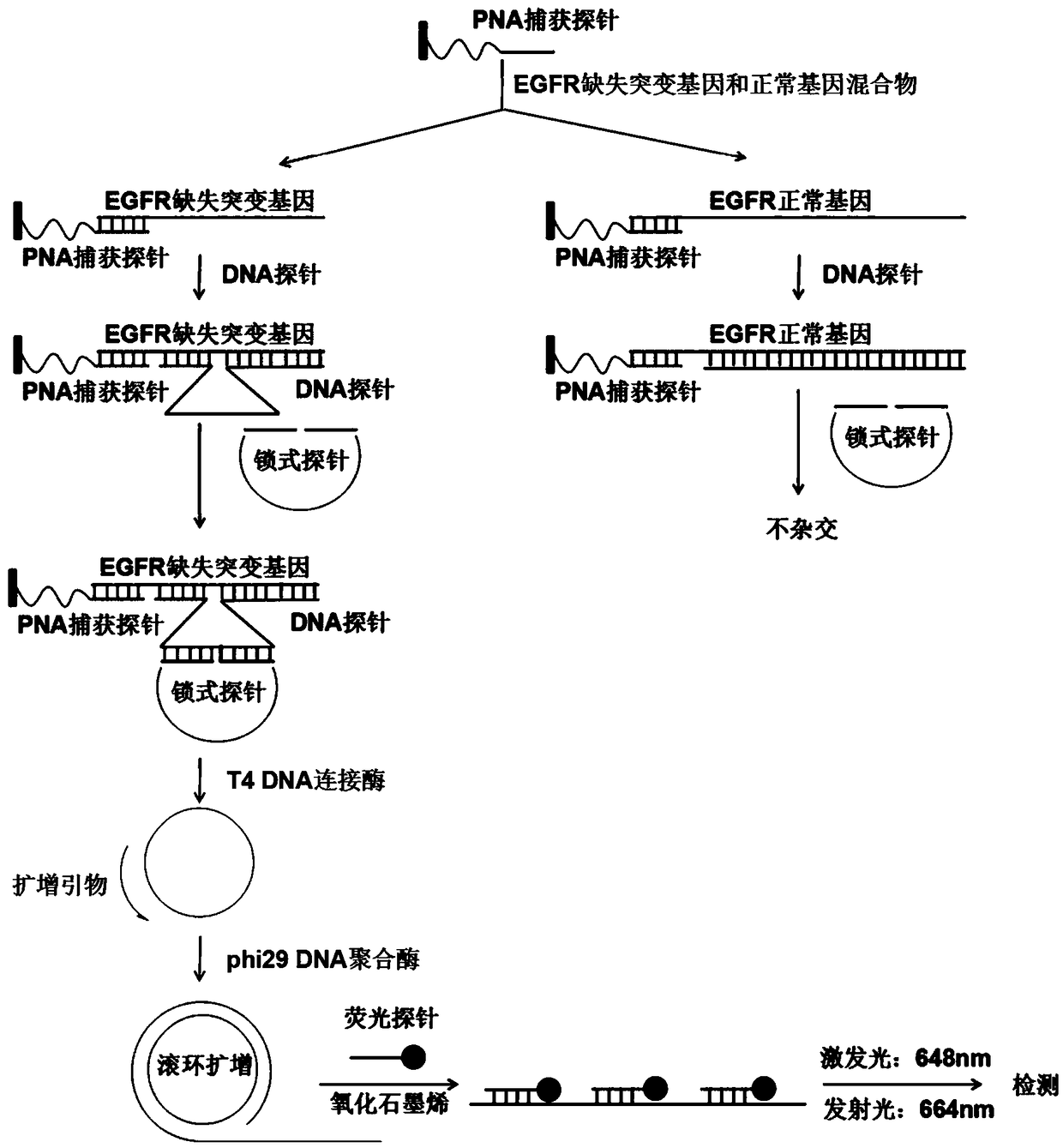
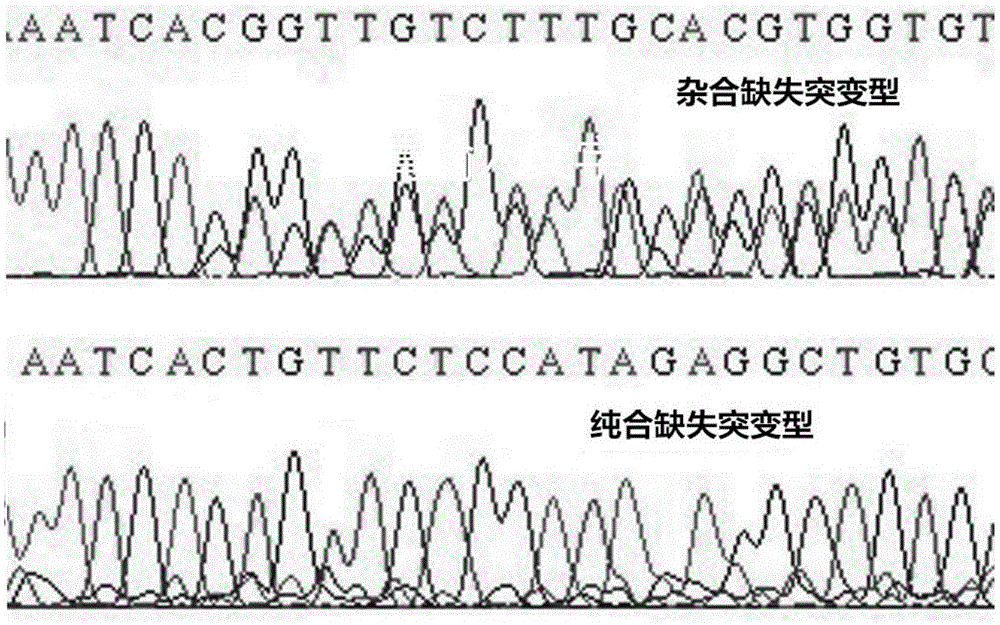
Fluorescence detection kit and fluorescence detection method for deletion mutation of gene
ActiveCN108949924AHigh sensitivityStrong specificityMicrobiological testing/measurementPolymerase LA-DNA
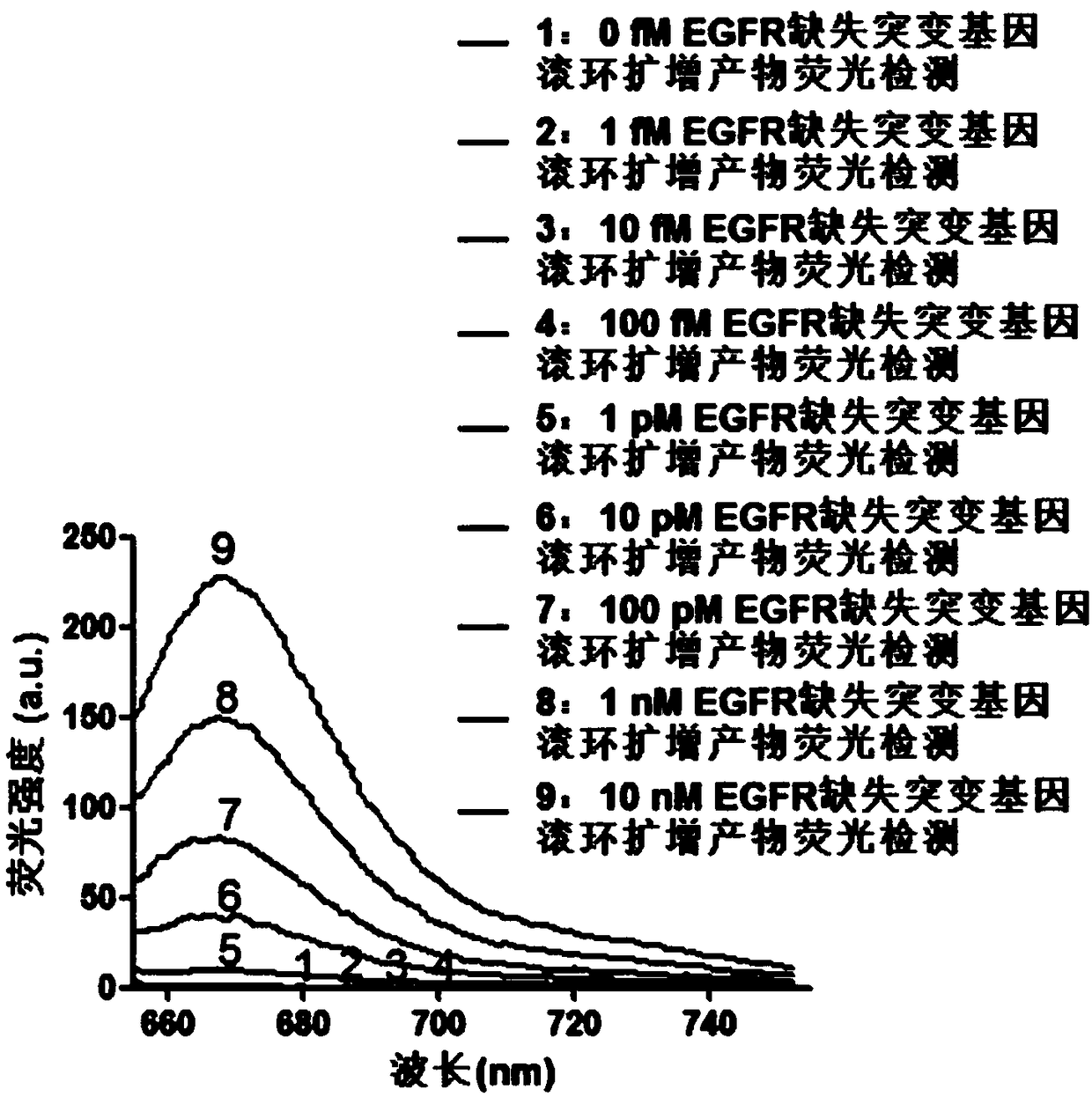
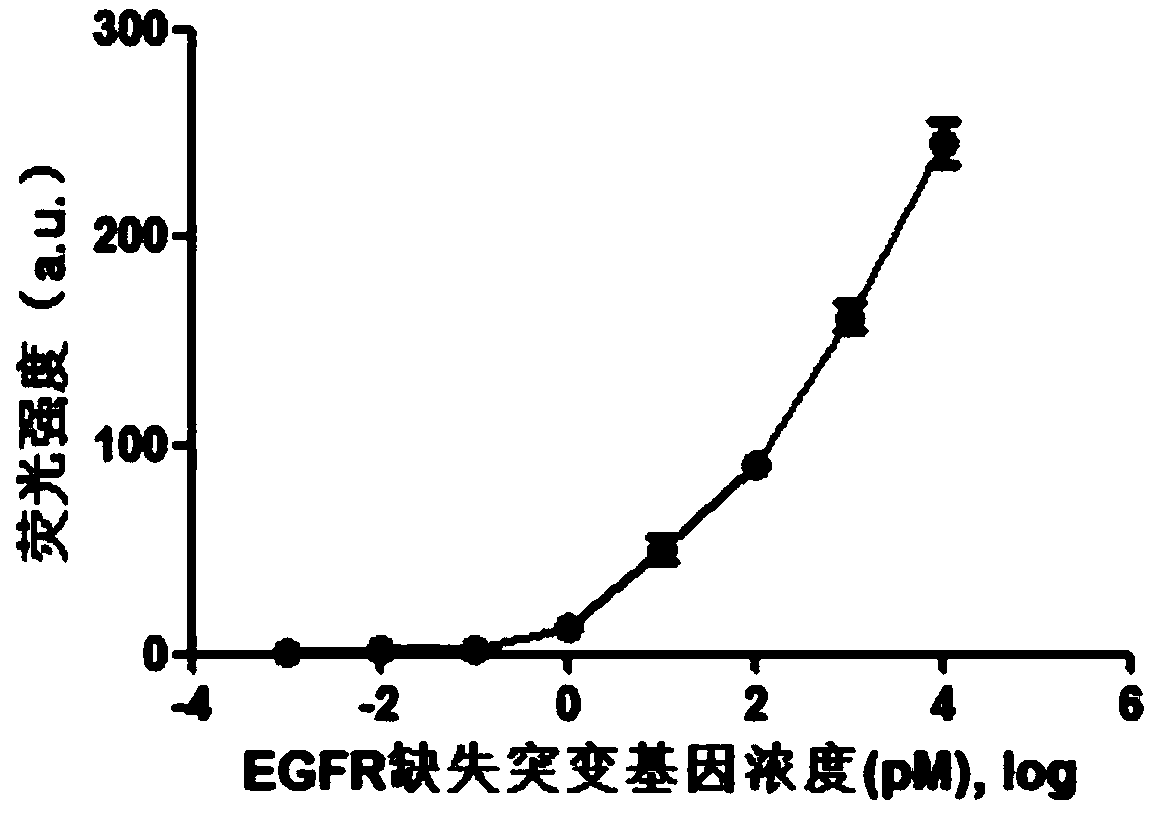
The invention provides a fluorescence detection kit for a deletion mutation gene. The kit includes: a PNA capture probe, a DNA probe, a padlock probe, a DNA polymerase, a DNA ligase, a rolling circleamplification primer, and a fluorescence probe. The invention also provides a method of using the kit to perform the fluorescence detection on the deletion mutation gene, wherein the method includes:1) immobilizing the PNA capture probe on the bottom of a pore plate, and hybridizing the probe with a target gene with a buffer solution; 2) hybridizing the target gene immobilized on the bottom of the pore plate with the DNA probe; 3) hybridizing a single chain section on the DNA probe, which is hybridized with the target gene, with the padlock probe, cyclizing the hybridized product with the DNAligase, and performing rolling circle amplification under effect of the rolling circle amplification primer and the DNA polymerase; 4) hybridizing the fluorescence probe with a rolling circle amplification product, quenching background fluorescence, and detecting the change on fluorescence intensity.
Owner:CIXI INST OF BIOMEDICAL ENG NINGBO INST OF MATERIALS TECH & ENG CHINESE ACAD OF SCI +1
Universal gene-knockout suicide vector for vibrios and application thereof
InactiveCN105063073AStrong lethal effectWide range of lethal objectsBacteriaHybrid cell preparationAgricultural scienceRestriction enzyme digestion
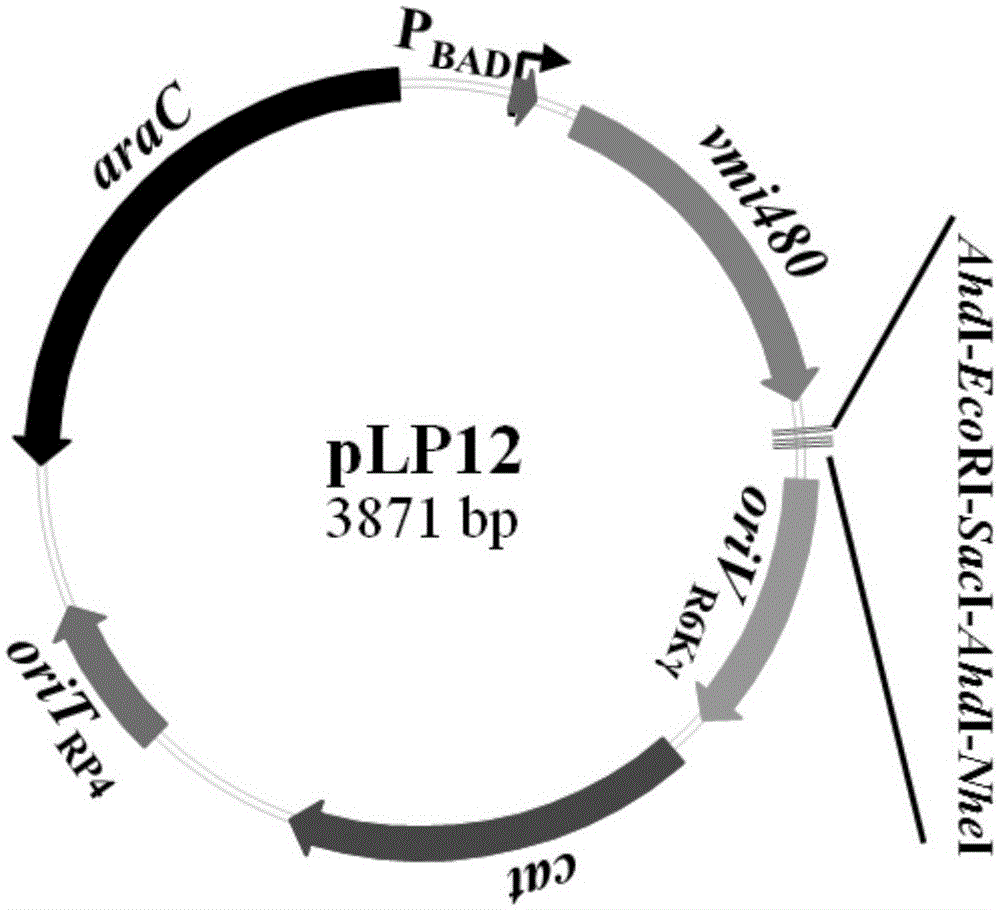
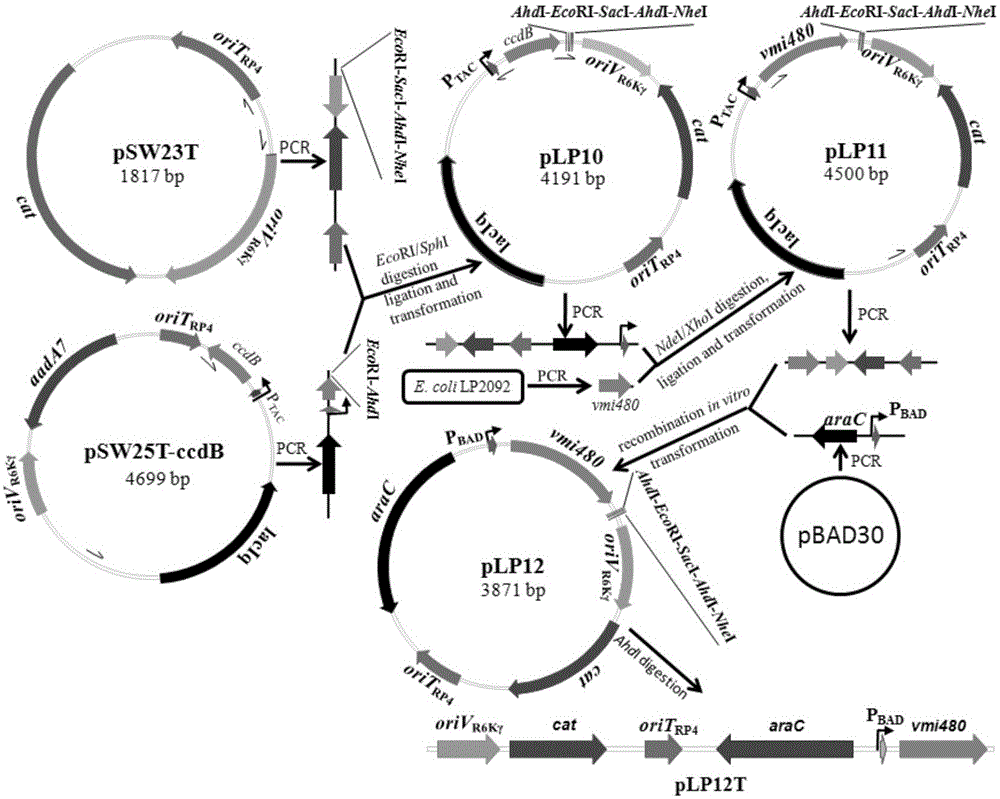
The invention discloses a universal gene-knockout suicide vector for vibrios and a construction method theroef and provides an application thereof in gene knockout of the vibrios. The universal gene-knockout suicide vector pLP12 is a ring-shaped vector and comprises a PBAD promoter, a repressor protein gene araC, an RP4 transferring initiation site, a chlorampenicol resistant gene, an R6K duplicating initiation site, a multiple-cloning-site area and a lethal gene vmi480; the multiple-cloning-site area at least contains two AhdI restriction enzyme digestion sites; the suicide vector pLP12 is subject to AhdI restriction enzyme digestion to form linearized suicide vector pLP12T. The universal gene-knockout suicide vector adopts entirely-new reverse selection genes vmi480 and is used for replacing the common sacB gene. Foreign fragments carried by the pLP12T are transferred to vibrio cells to be mutated by a jointing mode, under the pressure of antibiotics and reverse selection of products of lethal gene vmi480, first-time homologous recombination and second-time homologous recombination are carried out on the vibrios successively, and finally the mutant strain with deletion of target genes is generated.
Owner:SOUTH CHINA SEA INST OF OCEANOLOGY - CHINESE ACAD OF SCI
Watermelon oxysporum pathogenic FonAGL3 gene as well as deleted DNA fragment, deletion mutant and application thereof
InactiveCN107299105AClear control functionEffectiveness of Good Fusarium Wilt ControlBiocideFungiHygromycin BTreatment effect
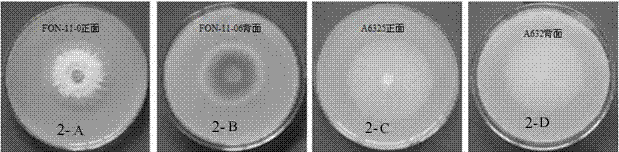
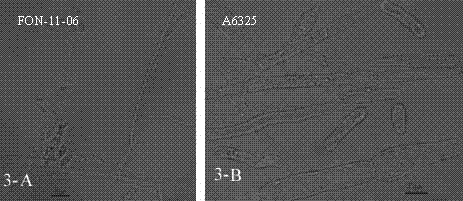
The invention discloses a watermelon oxysporum pathogenic FonAGL3 gene as well as a deleted DNA fragment, a deletion mutant and application thereof, and aims to solve the technical problem of biological prevention and treatment on watermelon oxysporum. A pathogenic gene FonAGL3 derived from watermelon fusarium oxysporum is analyzed and screened by inserting watermelon oxysporum T-DNA into a mutant library, by utilizing the homologous gene replacement principle, DNA fragments of the target gene FonAGL3 are replaced by gene DNA fragments of a resistance gene hygromycin B (HPH), a FonAGL3 gene deletion mutant is obtained by establishing a gene deletion carrier and implementing genetic transformation of a wild strain FON-11-06, and the FonAGL3 mutant bacterium has a good wilt prevention and treatment effect and is environmental-friendly and low in prevention and treatment cost.
Owner:河南省农业科学院园艺研究所 +1
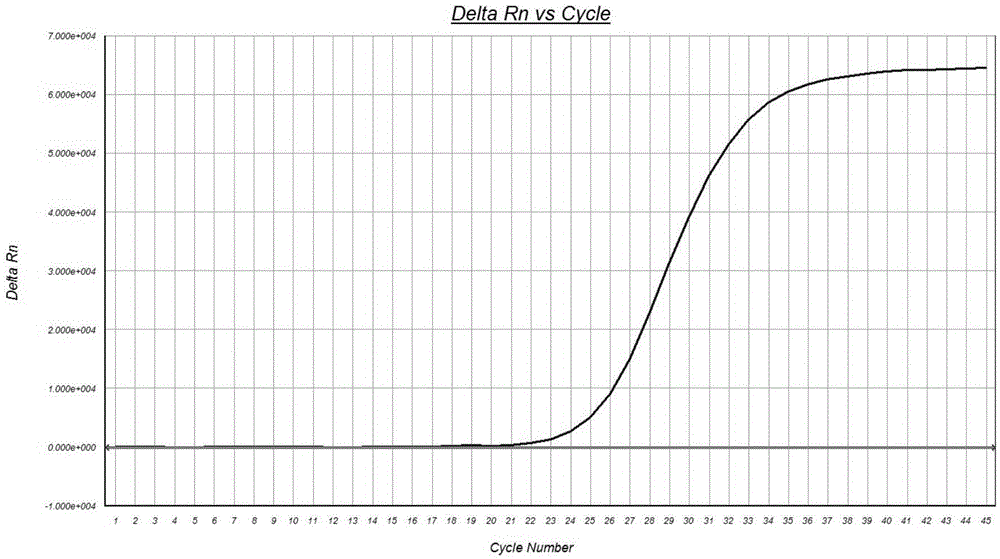
Digital PCR platform based gene deletion mutation detection method and kit thereof
InactiveCN103923973AHigh speedReduce false negativesMicrobiological testing/measurementWild typeGene
The invention relates to the field of the molecular biology, and concretely relates to gene deletion mutation detection method and a kit thereof. A PNA probe complementary to a wild non-mutation template sequence is added into a reaction system with digital PCR as a platform, and the repression effect of the PNA probe is utilized to make only deletion-mutated samples amplified; and the existence of mutant templates and the quantity and the proportion of the mutant templates are determined through fluorescence detection by utilizing a TaqMan probe as a fluorescent quantitative mark.
Owner:上海涌泰生物医药科技有限公司
Brucella molecule marking and virulence deletion attenuated vaccine and preparation method
InactiveCN101185756AWide application of practical valueAntibacterial agentsBacterial antigen ingredientsVirulent characteristicsVaccine Immunogenicity
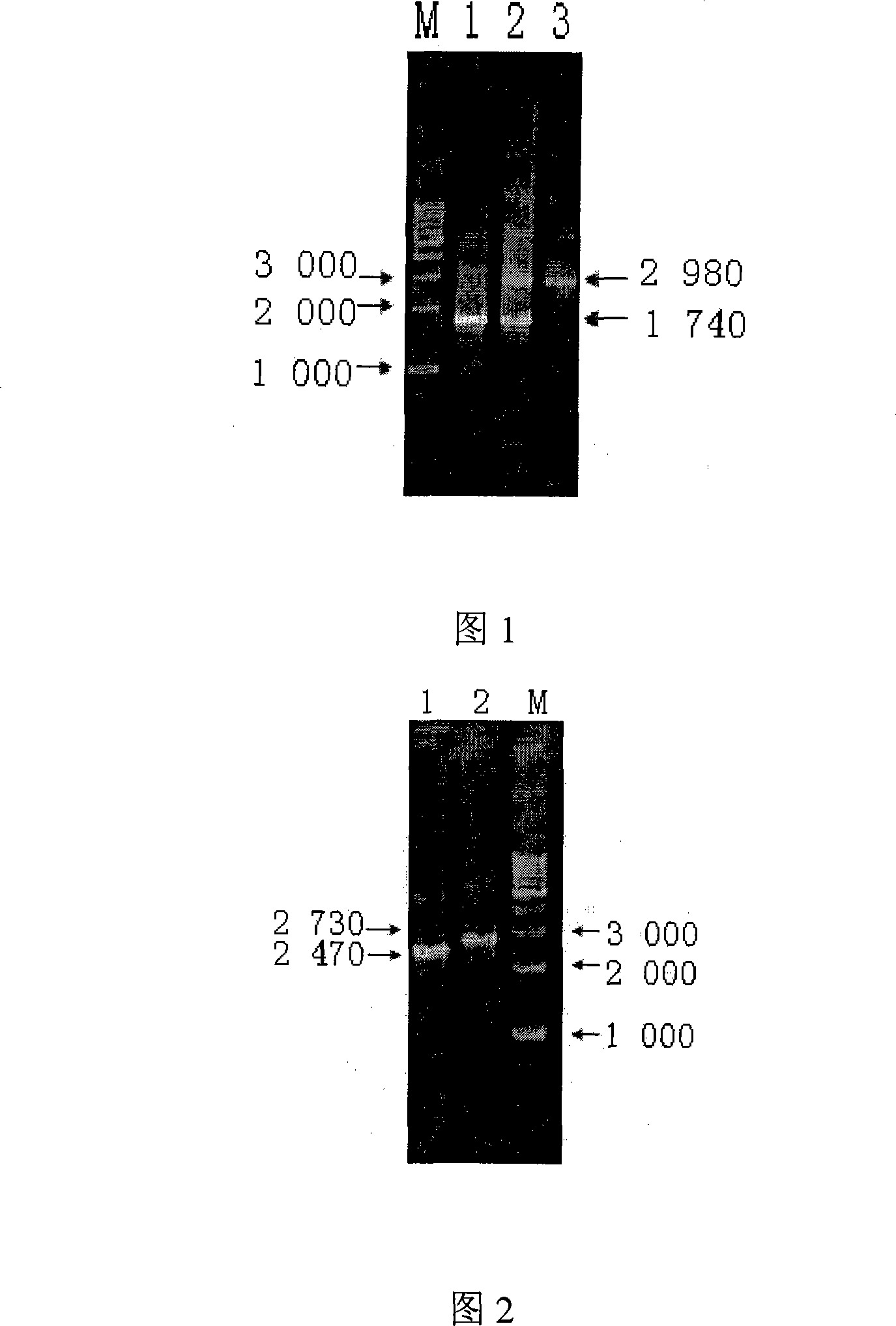
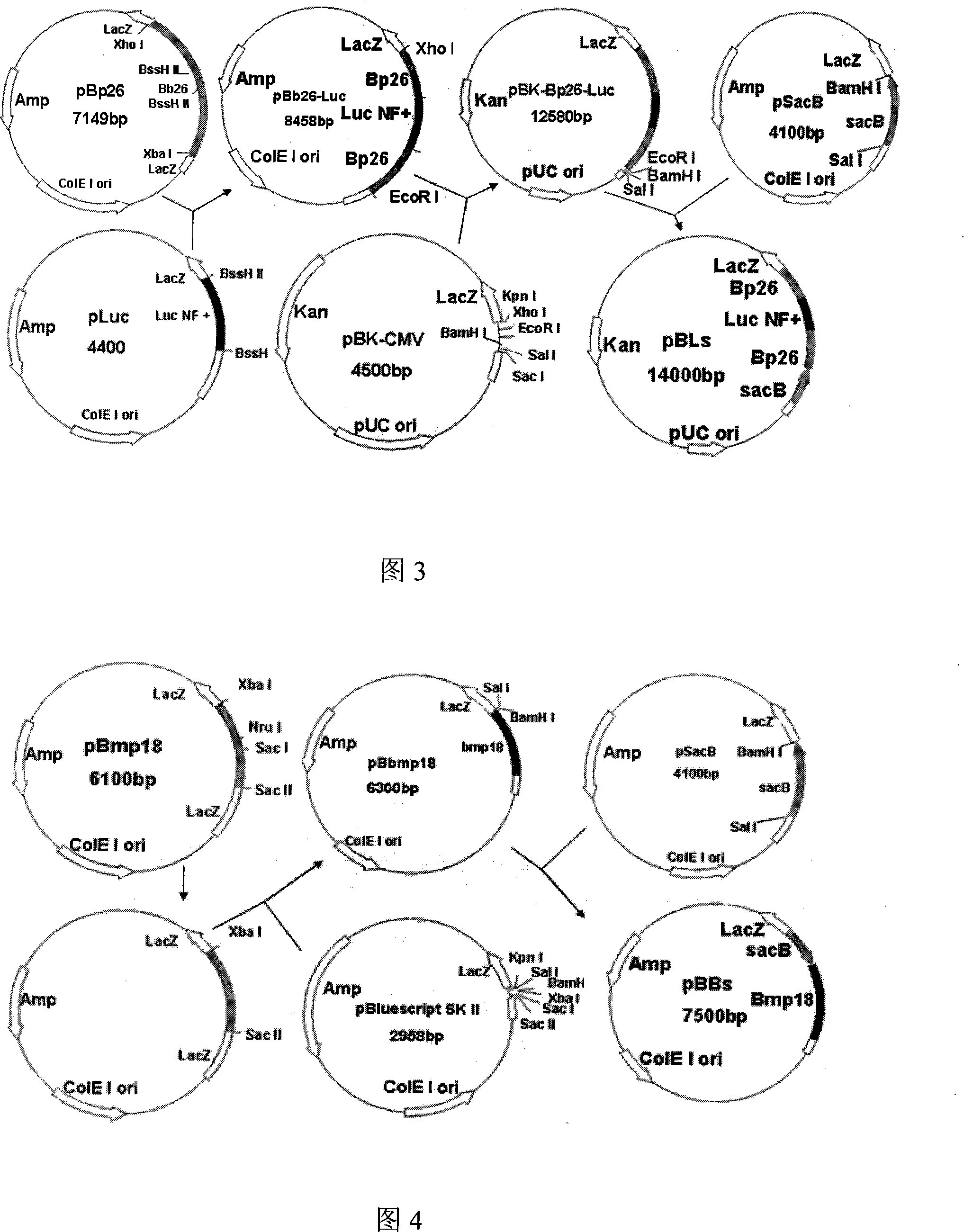
The invention relates to a Brucella vaccine, in particular to the molecular marker and virulence gene deletion of Brucella vaccine strain. The study uses luciferase modified gene (Luc NF plus) to replace the partial fragment of Bp26 gene of Brucella attenuated vaccine S19 strain by constructing suicide plasmid and adopting the method of targeted homologous recombination (gene targeting), so as to damage the expression of the immunogenicity protein BP26 and construct the gene deletion mutant strain Delta S19-1 of the Brucella Bp26. The BMP18 protein is one of the main virulence factors of Brucella. The invention adopts the same method to exclude the Bmp 18 gene of Delta S19-1, so as to lead the Delta S19-1 not to express the Bmp 18 protein and the Brucella virulence gene deletion mutant strain Delta S19-2 is constructed. The invention solves the problems that the conventional Brucella vaccine can not distinguish between the artificial immunization and the wild bacteria infection of people and animals, the virus is strong and the vaccine is easy to cause the illness of inoculated people and animals. The invention has important significance and practical application value of the monitoring, diagnosis, purification and all the controls of Brucella.
Owner:MILITARY VETERINARY RES INST PLA MILITARY MEDICAL ACAD OF SCI
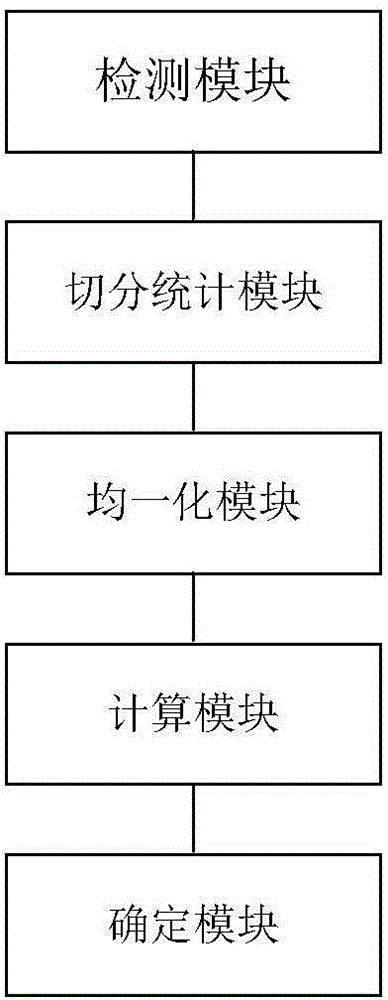
Detection method and device of gene deletion mutation
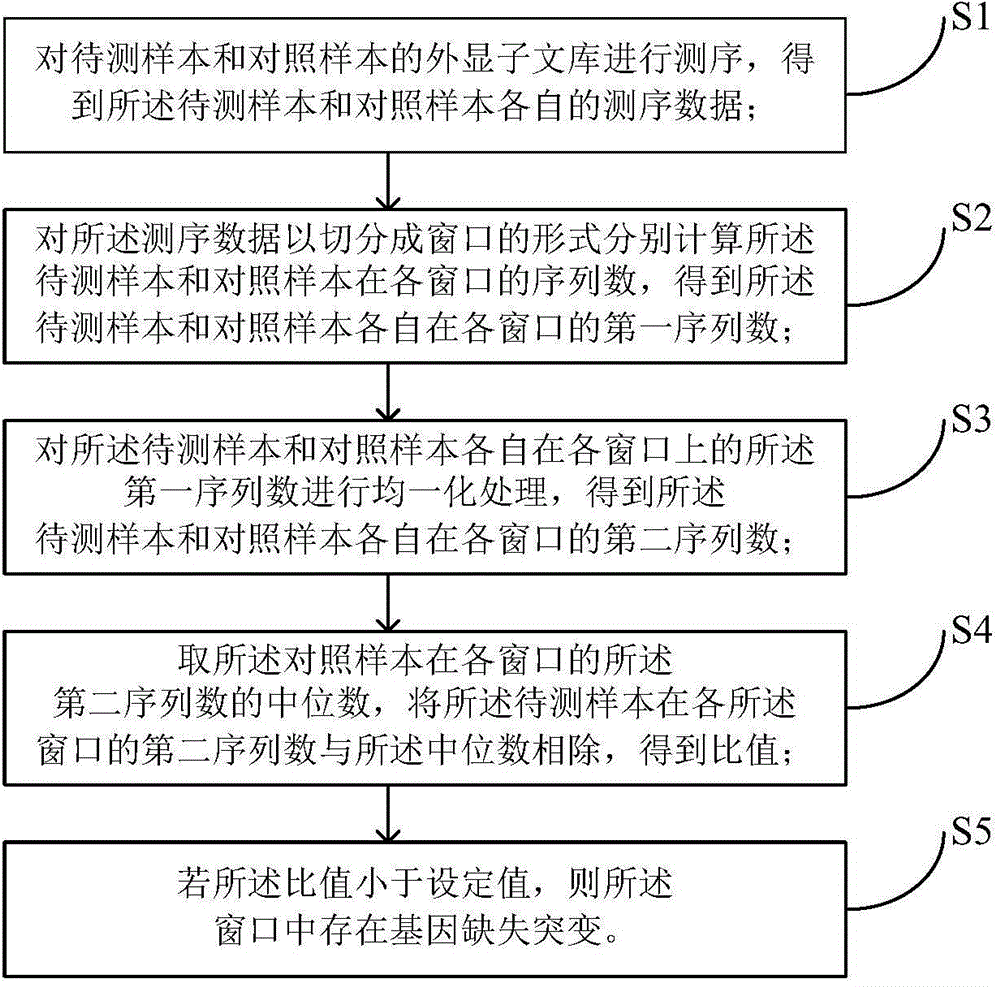
InactiveCN104561289AWide range of sizesFlexible Size SlicingBioreactor/fermenter combinationsSequential/parallel process reactionsExonComparison standard
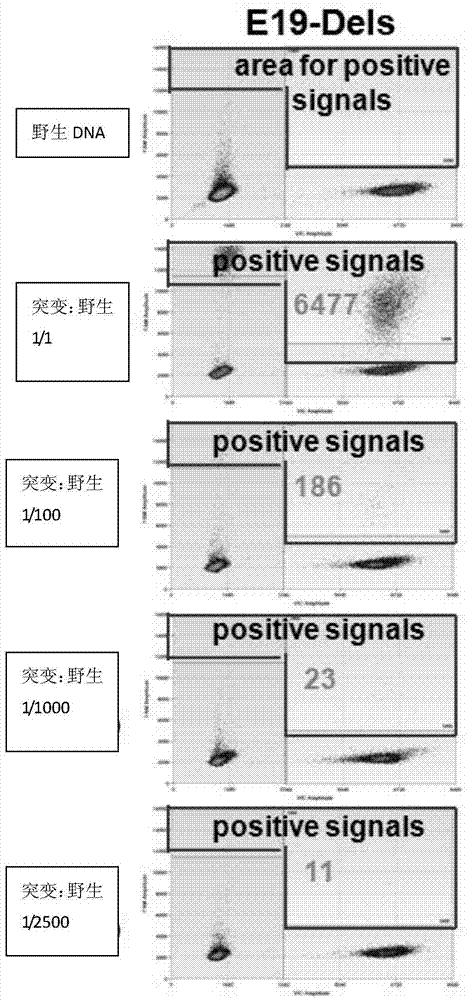
The invention discloses a detection method and a detection device of gene deletion mutation. The method comprises the following steps: sequencing exon libraries of a sample to be detected and a comparison sample to acquire sequencing data of the sample to be detected and the comparison sample; respectively calculating sequencing numbers of the sample to be detected and the comparison sample on all windows in a form of dividing the sequencing data into several windows to acquire first sequencing numbers of the sample to be detected and the comparison sample on all the windows; performing homogenization on the first sequencing numbers of the sample to be detected and the comparison sample on all the windows to acquire second sequencing numbers of the sample to be detected and the comparison sample on all windows; dividing the second sequencing number of the sample to be detected on the windows by a median which is acquired from the second sequencing number of the comparison sample on all the windows to acquire a specific value; performing gene deletion mutation in the windows if the specific value is smaller than a set value. According to the method, the median in the comparison sample is used as a comparison standard, and compared with an average value or a standard difference, false positive can be relatively easily differentiated, and the result is relatively accurate.
Owner:BEIJING NOVOGENE TECH CO LTD +1
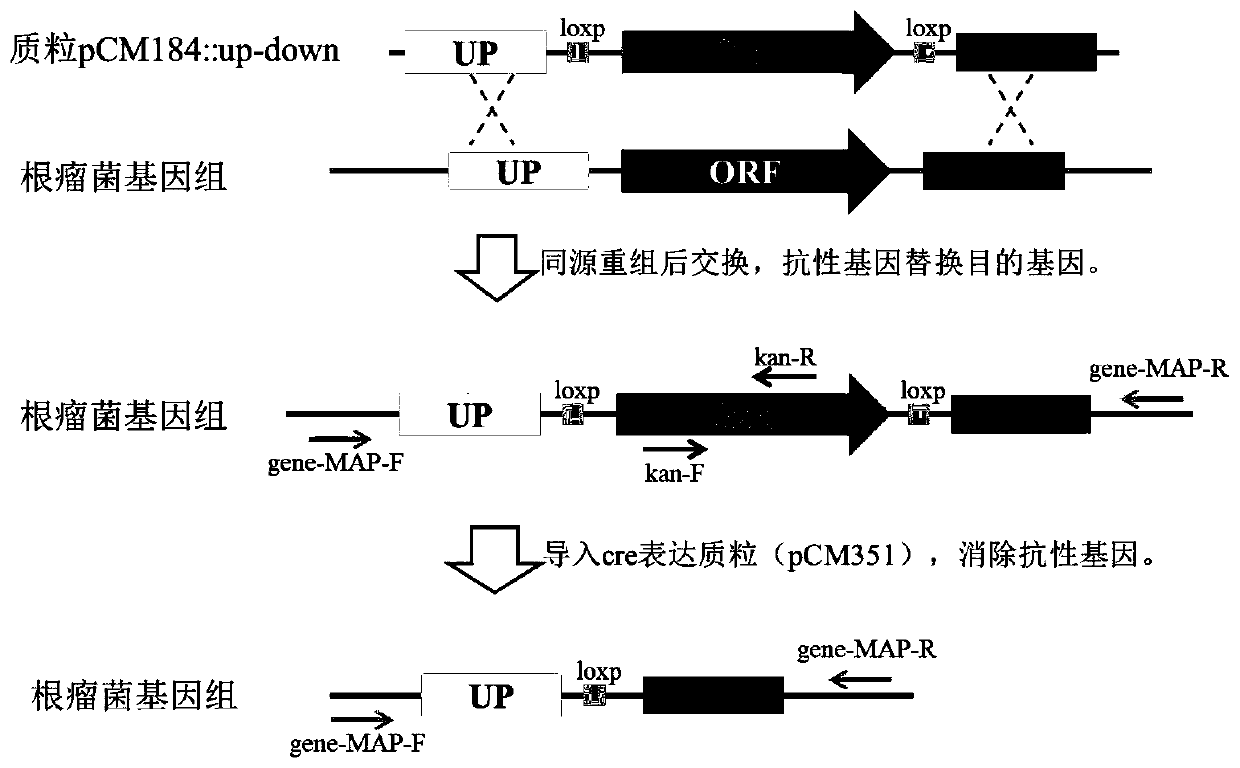
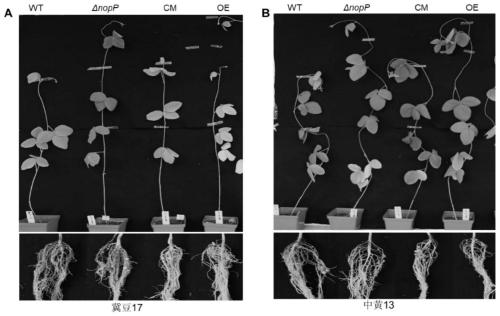
Mutant of rhizobium japonicum SMH12 and application of mutant
ActiveCN111286513ANodulation Restriction LiftedDecreased defense responseBiocidePlant growth regulatorsBiotechnologyEnzyme digestion
The invention discloses a mutant of rhizobium japonicum SMH12 and application of the mutant. The nopP gene deletion mutant is constructed by utilizing a CreloxP system double-exchange replacement method. By taking fast-growing rhizobium SMH12 genome DNA as a template and adopting a nopP-up-F / R primer pair and a nopP-down-F / R primer pair, a nopP homologous exchange upper arm and a nopP homologous exchange lower arm are respectively amplified, a product is connected with a digested pCM351 vector in two steps after being subjected to double enzyme digestion, plasmid transformation is obtained, and the mutant can be obtained. Therefore, the rhizobium target gene mutant is constructed and obtained. After the nopP gene is mutated or deleted, the defense reaction of leguminous plants is inhibitedor eliminated, so that rhizobium infection and nitrogen fixation are promoted. The improved rhizobium can improve the growth and nitrogen fixation capacity of leguminous crops, reduce nitrogen fertilizer application in agricultural production and generate huge economic and ecological benefits.
Owner:华创佳农生物科技(武汉)有限公司
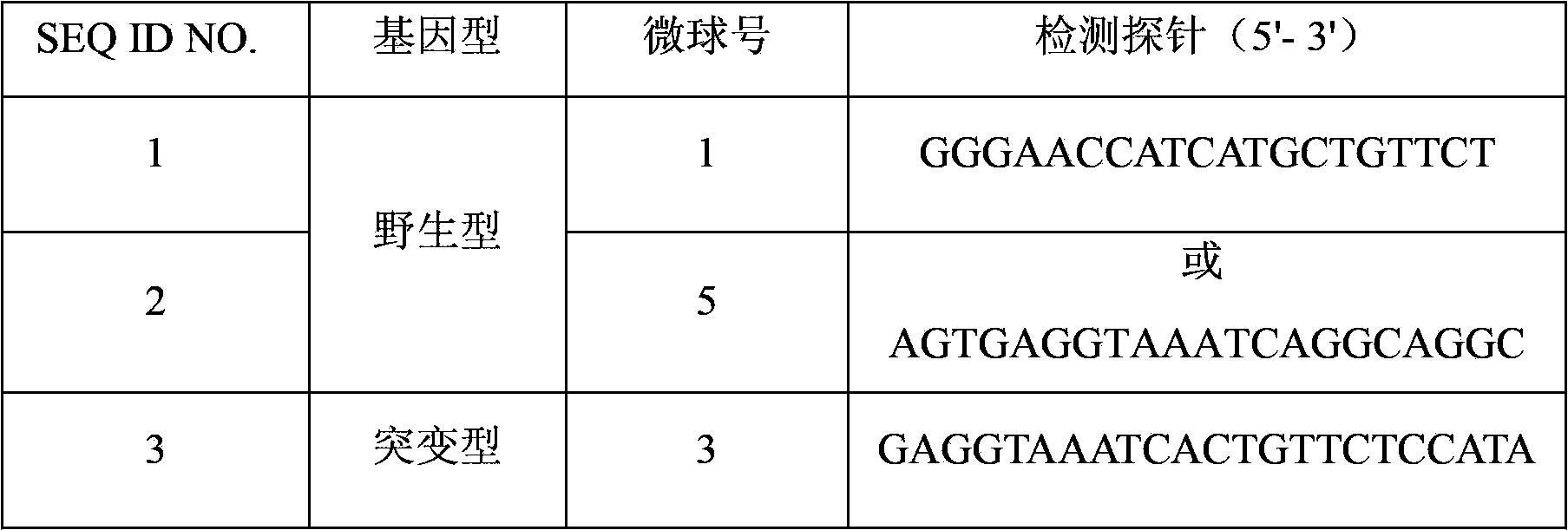
Detection probes and detection liquid phase chip for BIM gene deletion mutation
ActiveCN103571923AImprove signal-to-noise ratioNo cross-reactivityMicrobiological testing/measurementDNA/RNA fragmentationGenes mutationMicrosphere
The present invention discloses a detection liquid phase chip and detection probes for BIM gene deletion mutation. The detection liquid phase chip comprises: BIM gene deletion mutation wild-type targeted detection probe coated microspheres and BIM gene deletion mutation mutant-type targeted detection probe coated microspheres, wherein the wild-type detection probe is SEQ ID NO.1 or SEQ ID NO.2, and the mutant-type detection probe is SEQ ID NO.3 or SEQ ID NO.4; and biotin labeled amplification primers. According to the present invention, the prepared BIM gene mutation detection liquid phase chip has a good signal-to-noise ratio, no cross-reaction basically exist among the designed detection probes, the amplification primers and the amplification product, and parallel detection on the wild-type sequence and the mutant-type sequence in the same reaction system can be achieved.
Owner:SUREXAM BIO TECH
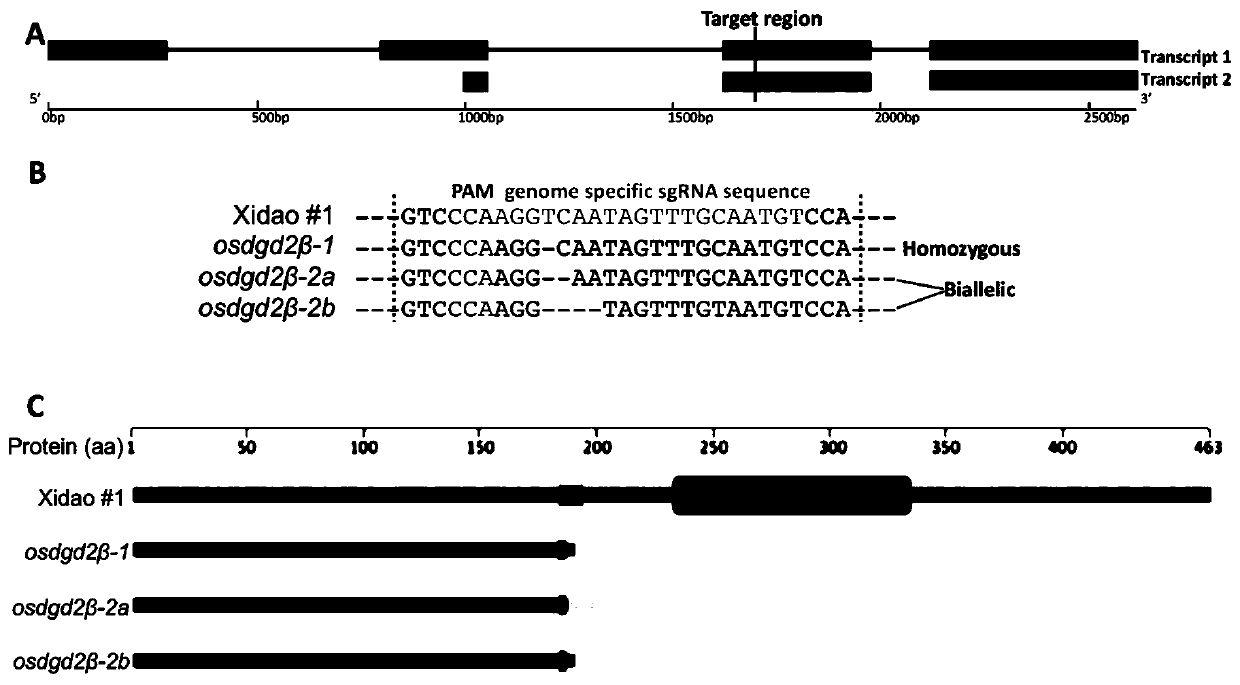
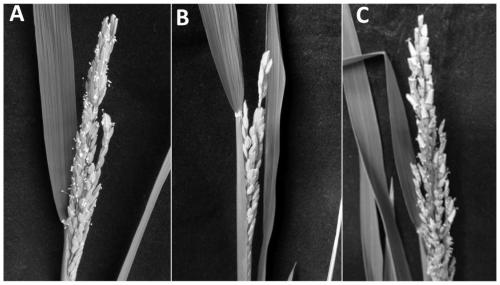
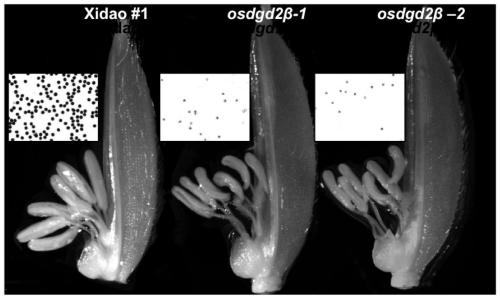
Application of OsDGD2beta gene in cultivating male sterile rice varieties
Owner:ZHEJIANG UNIV
Method for constructing pseudorabies virus gene-deleted attenuated strain and application of attenuated strain
ActiveCN111635891AReduce immunosuppressionViral antigen ingredientsTransferasesDeletion mutationPseudorabies
The invention provides a method for constructing a pseudorabies virus gene-deleted attenuated strain, a recombinant virus constructed by the method and application. According to the method, a pseudorabies virus gene-deleted attenuated strain is constructed by utilizing a CRISPR / Cas9 gene editing technology, and the pseudorabies virus gene-deleted attenuated strain constructed by the method is subjected to specific deletion mutation at a UL13 gene part. The expression quantity of the I-type IFN gene induced by the obtained pseudorabies virus gene-deleted attenuated strain is remarkably increased, a stronger natural immune reaction can be generated, and the replication of the virus is not remarkably different. Therefore, the UL13 gene deletion mutation can significantly reduce immunosuppression caused by the pseudorabies virus, and has important potential application value.
Owner:SHANDONG AGRICULTURAL UNIVERSITY
Method for improving acid stress resistance of candida glabrata
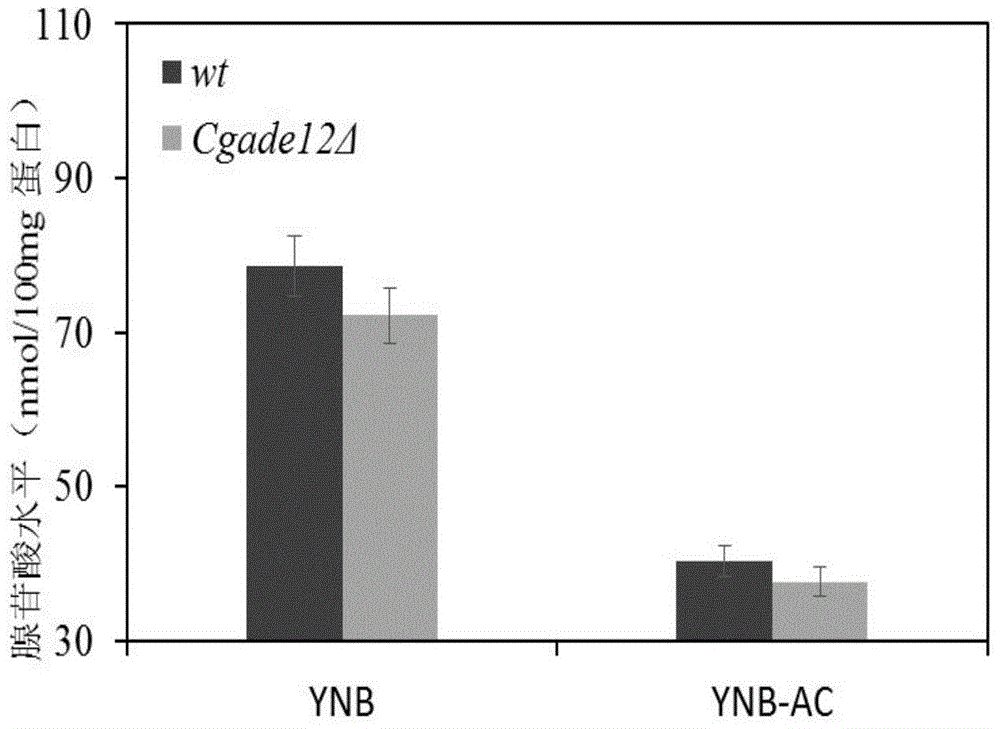
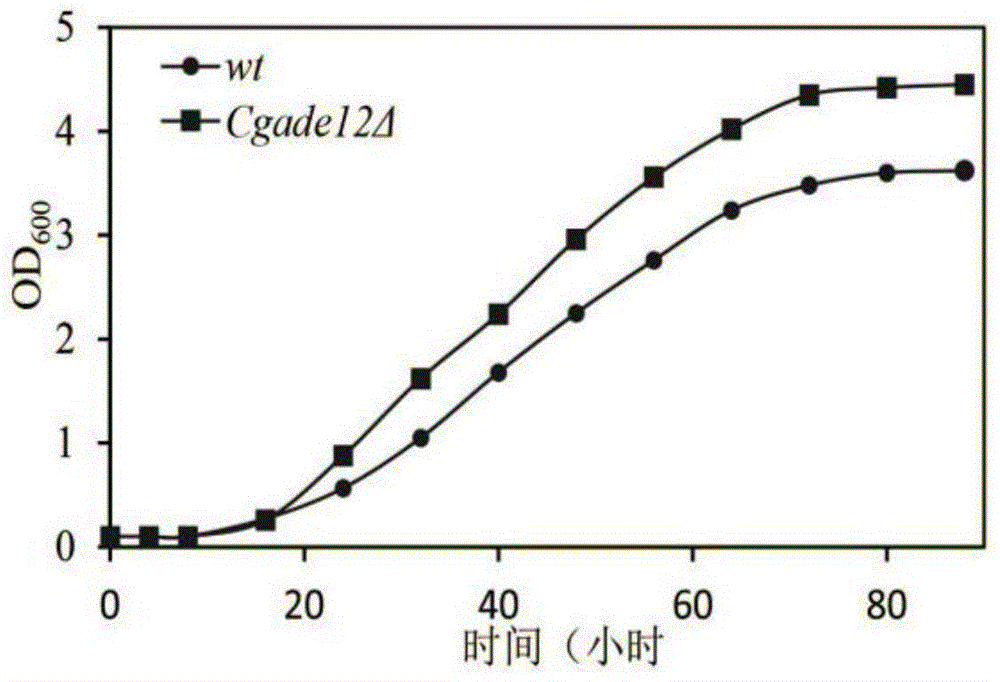
ActiveCN104789600AImprove growth performanceImprove acid stress resistanceFungiStable introduction of DNAAcetic acidCandida glabrata
The invention discloses a method for improving the acid stress resistance of candida glabrata, and belongs to the field of bioengineering. The method comprises the following steps: improving the candida glabrata, constructing a gene-deleted mutant strain Cgade12Delta, measuring the intracellular ATP level of the constructed strain, and determining the organic acid tolerance of the strain by a plate growth experiment and cellular activity analysis. After the deletion of the gene CgADE12 is found out, the intracellular ATP level of the strain under the stress of 0.2 percent acetic acid is lowered, the growth capability under the stress of acetic acid is improved, and the acetic acid tolerance is improved.
Owner:JIANGNAN UNIV
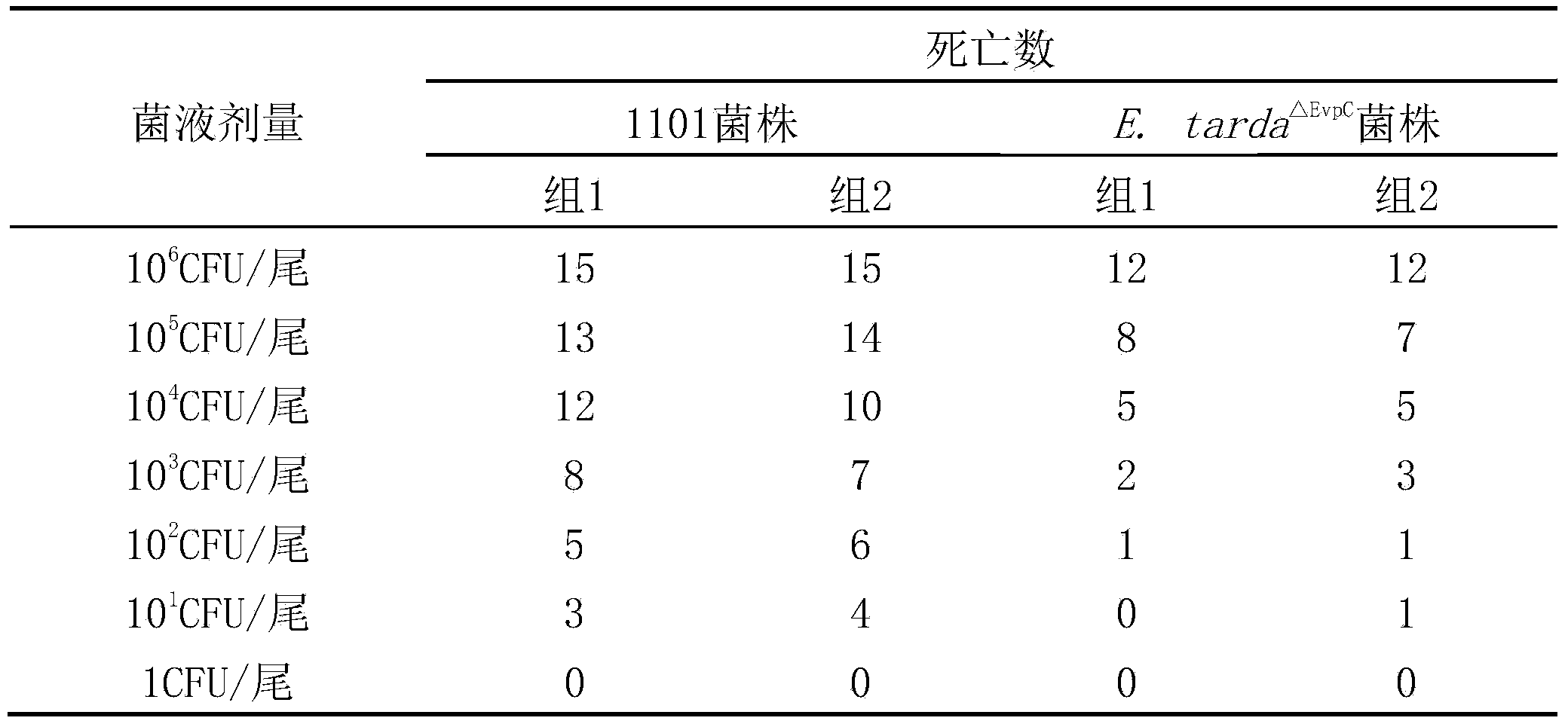
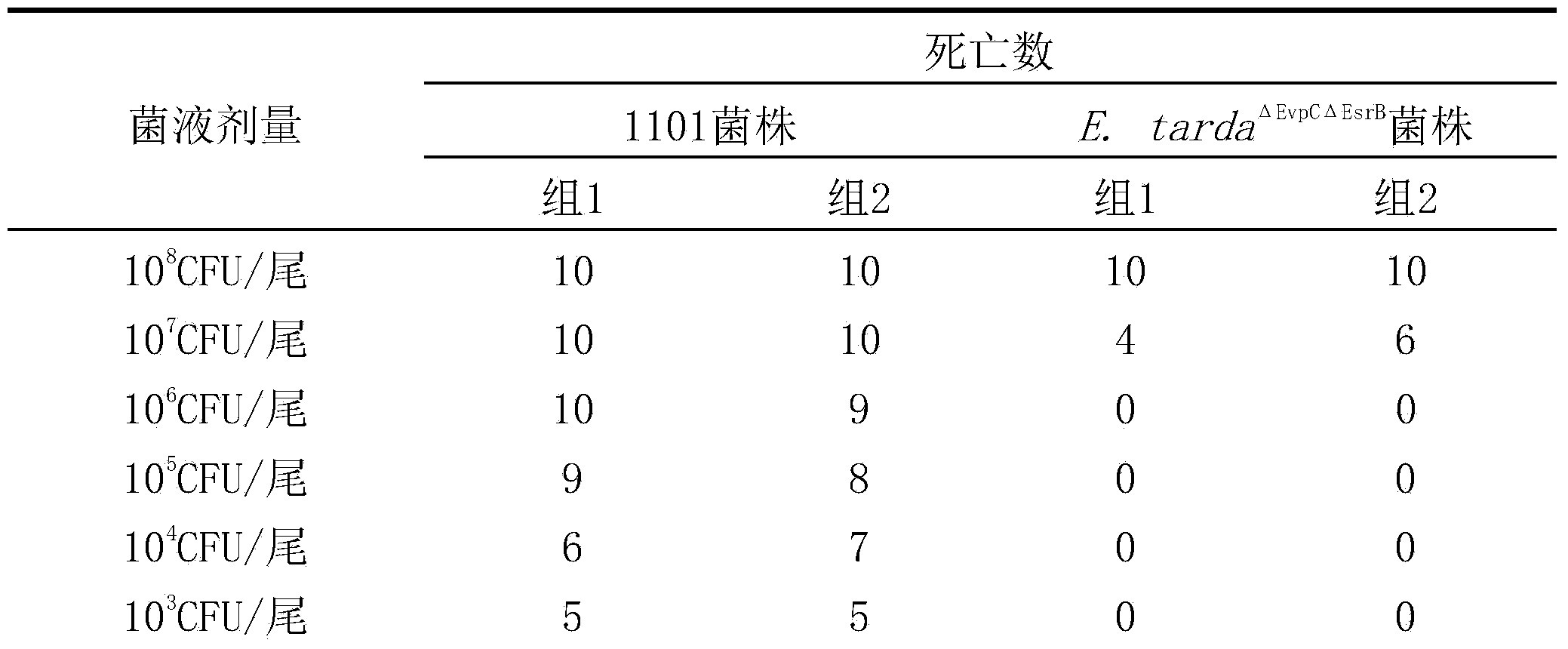
Genetically engineered attenuated strains of edwardsiella tarda (E.tarda) and application thereof
ActiveCN103667145AGood immune protectionReduce atavismAntibacterial agentsBacterial antigen ingredientsVirulent characteristicsEdwardsiella
The invention provides four gene deletion mutants of wild edwardsiella tarda (E.tarda) and derivative strains thereof. The four gene deletion mutants are respectively an E.tarda<deltaEvpC> strain with an EvpC gene losing function and derivative strains thereof, an E.tarda<deltaEvpCdeltaEsrB> strain with an EsrB gene losing function and derivative strains thereof, an E.tarda<deltaEvpCdeltaEsrBdeltaPstB> strain with a PstB gene losing function and derivative strains thereof, and an E.tarda<deltaEvpCdeltaEsrBdeltaPstC> strain with a PstC gene losing function and derivative strains thereof. 1-3 genes such as EvpC, EsrB, PstB, PstC and the like of the attenuated strains have parts causing loss of function or suffer from complete deletion, point mutation, shifting or insertion. Compared with a wild 1101 strain or other virulent strains, the E.tarda<deltaEvpC> strain, the E.tarda<deltaEvpCdeltaEsrB> strain, the E.tarda<deltaEvpCdeltaEsrBdeltaPstB> strain and the E.tarda<deltaEvpCdeltaEsrBdeltaPstC> strain have the advantage that the virulence does not exceed 1 / 40, 1 / 400, 1 / 4000 and 1 / 4000 of the virulence of the wild E.tarda 1101 strain or other virulent strains. The genetically engineered attenuated strains of E.tarda can be applied as attenuated vaccine strains of E.tarda, genetic engineering vectors or probiotics.
Owner:YELLOW SEA FISHERIES RES INST CHINESE ACAD OF FISHERIES SCI
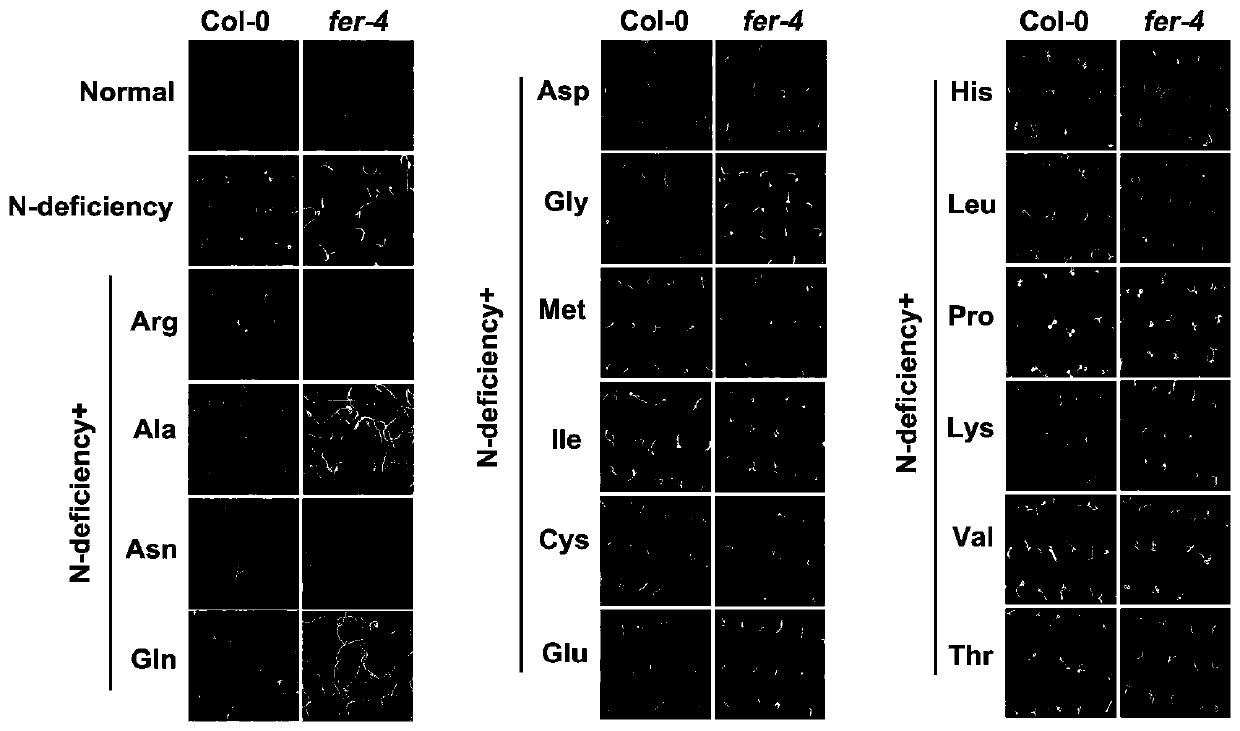
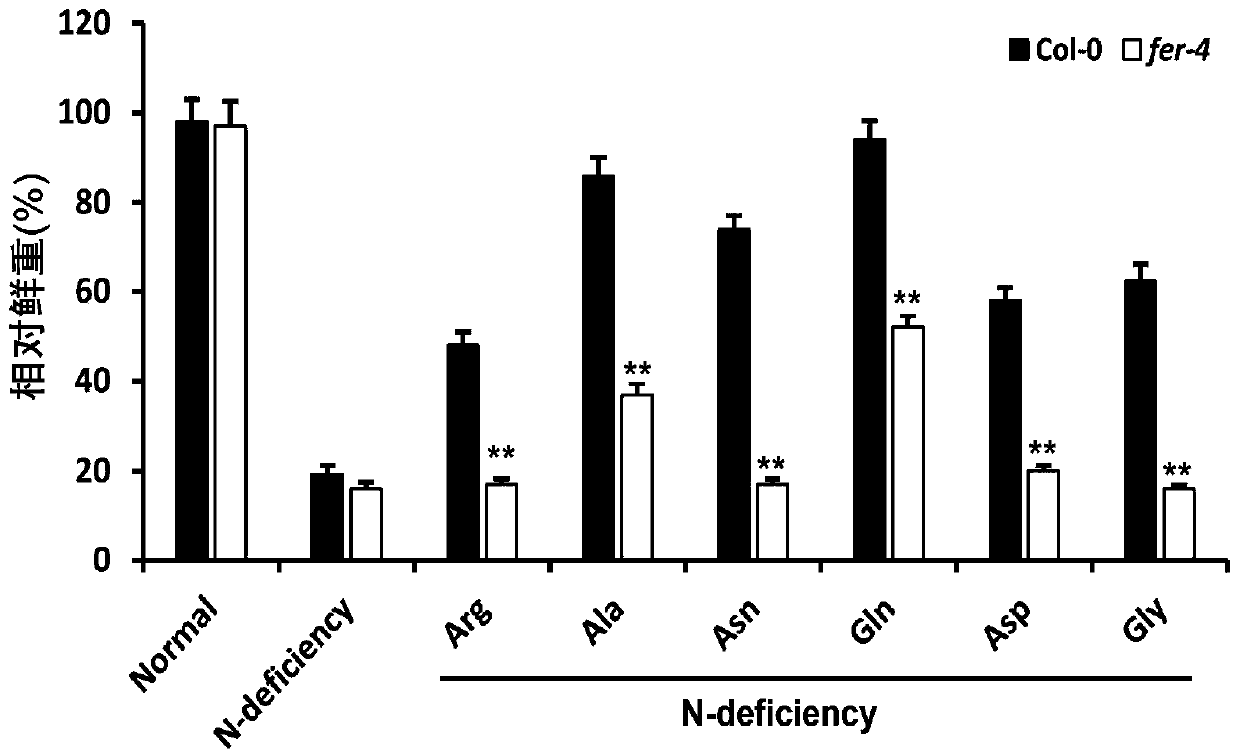
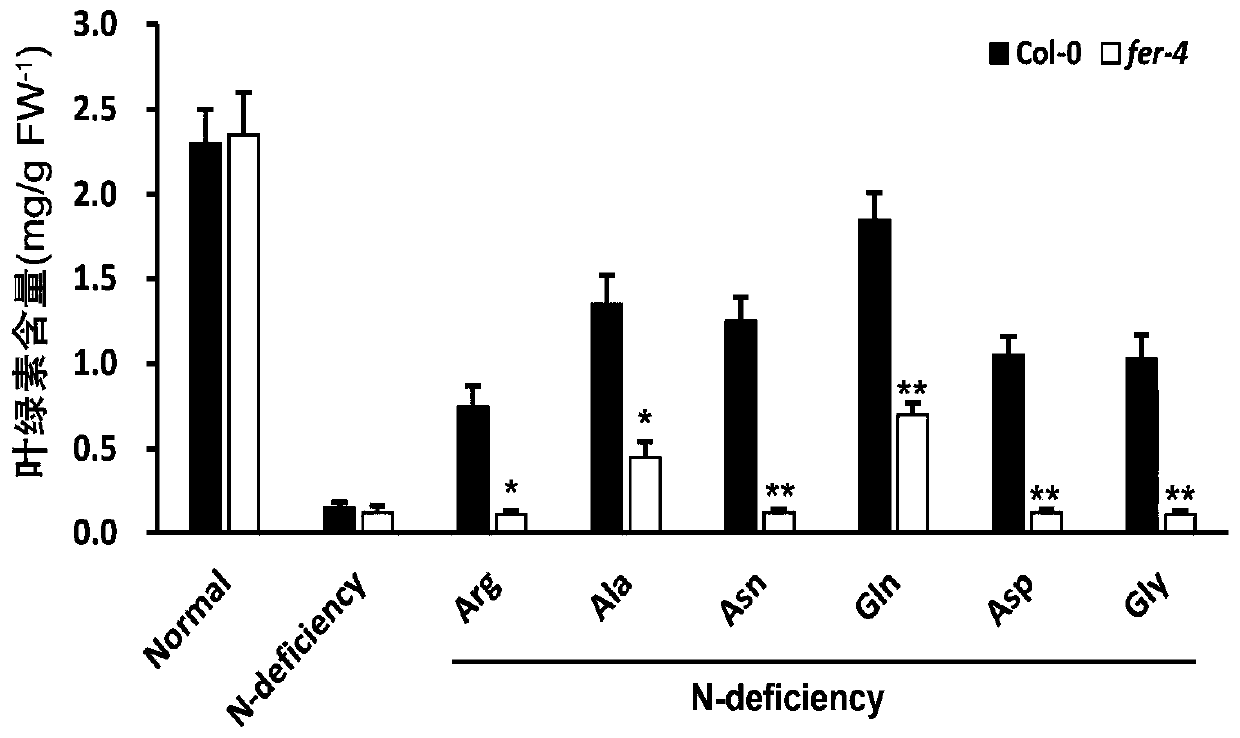
Application of gene FERONIA
The invention discloses an application of a gene FERONIA, and belongs to the technical field of gene regulation and control. The invention discloses a method for adding amino acid into a nitrogen-deficient culture medium. Wild type arabidopsis thaliana Col-0 and FERONIA gene deletion mutants fer-4 are dibbled on a culture medium of the arabidopsis thaliana; amino acid capable of restoring the nitrogen deficiency phenotype of wild arabidopsis thaliana is found, but the nitrogen deficiency phenotype of the arabidopsis thaliana fer-4 mutant cannot be restored or only part of the amino acid can berestored, and it is shown that FERONIA participates in the utilization process of organic nitrogen by plants, that is, the FERONIA gene of arabidopsis thaliana participates in the response process ofplant roots. The invention provides a theoretical and method basis for functional research of the FERONIA gene. The FERONIA disclosed by the invention can provide theoretical basis and genetic resources for cultivating new crop varieties.
Owner:HUNAN UNIV
Trichoderma phospholipase A2 and gene for expressing thereof
The invention relates to a trichoderma phospholipase A2 and a gene for expressing the same. The invention belongs to the field of agricultural biotechnology. The invention provides sequences of the trichoderma phospholipase A2 (PLA2) amino acid and nucleotide, and constructs efficient prokaryotic expression of the trichoderma phospholipase A2 and purifies system; the invention constructs deletion mutants of the gene, and uses the gene to prevent and control the curvularia leaf spot and achieves good effect. The invention firstly gains the trichoderma phospholipase A2 from filamentous fungi, and clones the gene which expresses the same. The efficient method for gaining the trichoderma phospholipase A2 provides the possibility of producing enzyme preparation. The trichoderma phospholipase A2 has double functions of both esterase and phospholipase A2, which indicates the trichoderma phospholipase A2 has good application value in industrial catalysis; and the function of the deletion mutants of the gene on preventing and controlling the curvularia leaf spot provides new application, and can greatly reduce curvularia leaf spot disease to improve maize yield.
Owner:SHANGHAI JIAO TONG UNIV
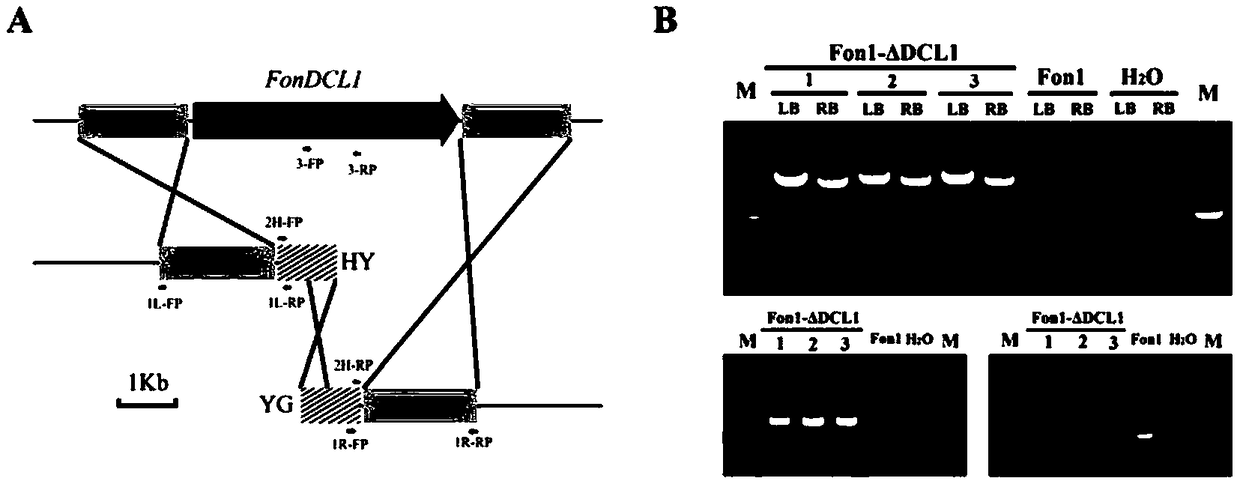
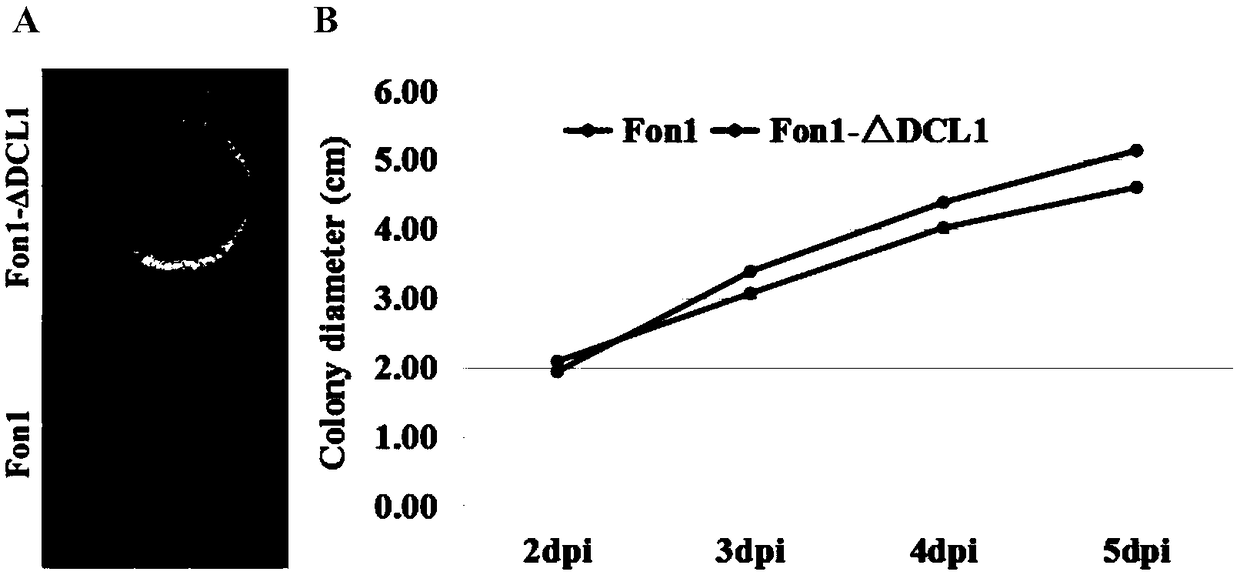
Fusarium oxysporum f.sp.niveum RNAi component FonDCL1 gene deletion mutant and construction method thereof
The invention discloses a fusarium oxysporum f.sp.niveum RNAi component FonDCL1 gene deletion mutant and a construction method thereof. According to the fusarium oxysporum f.sp.niveum RNAi component FonDCL1 gene deletion mutant and the construction method thereof, according to a reported Dicer like 1 protein sequence, homologous alignment is conducted on a Fon genomic sequence to obtain a fusariumoxysporum f.sp.niveum FonDCL1 gene, by adopting a homologous gene substitution principle, by means of a Split-marker strategy, the target gene FonDCL1 is substituted for a hygromycin B (HPH) gene DNAfragment, and by constructing a homologous gene recombinant fragment, through genetic transformation of a wild strain Fon1, the FonDCL1 gene deletion mutant is obtained. The FonDCL1 mutant has the advantages that the virulence to watermelon seedlings is enhanced, and the FonDCL1 mutant is more sensitive to abiotic stress factors of monensin sodium salt, hydroxycarbamide, a fluorescent whitening agent CFW, Congo red (CR), hydrogen peroxide and the like, so that a research foundation is provided for functional assignment of small RNA generated in processes of self growth and development and watermelon infection of the Fon1.
Owner:ENVIRONMENT & PLANT PROTECTION INST CHINESE ACADEMY OF TROPICAL AGRI SCI
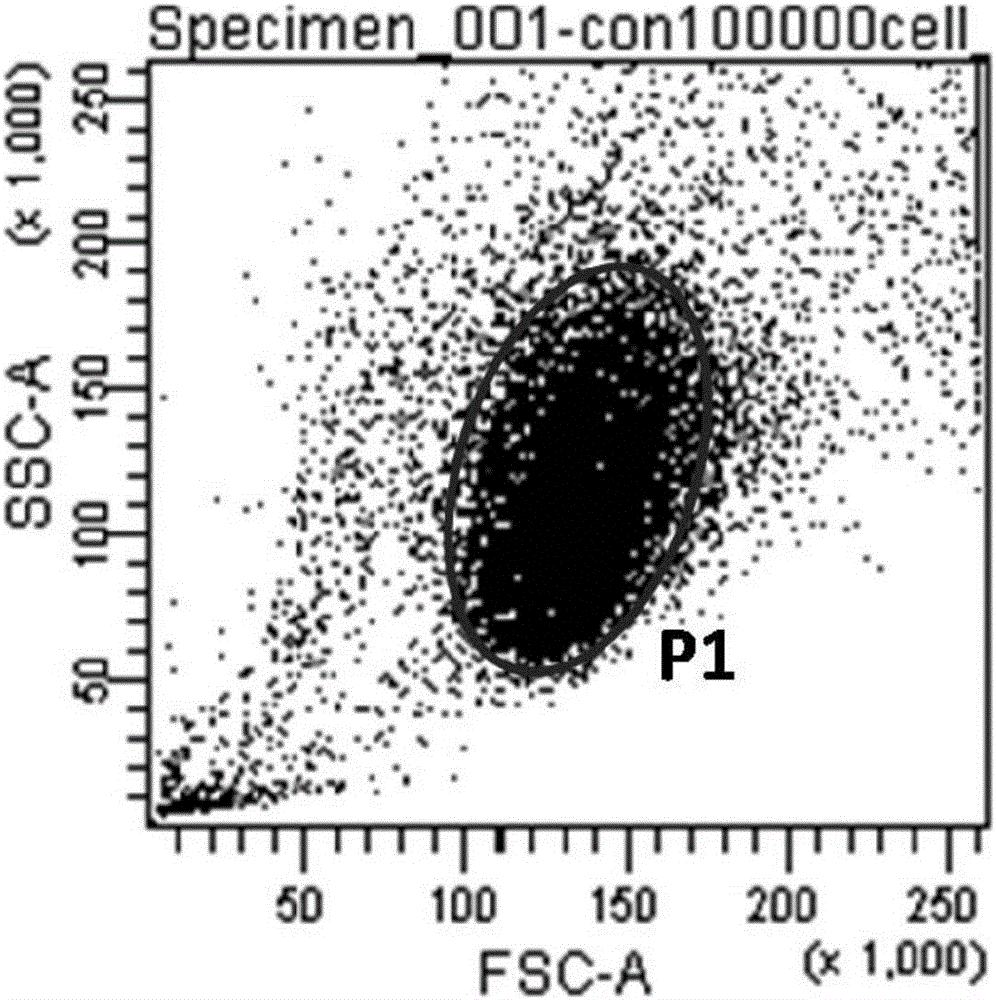
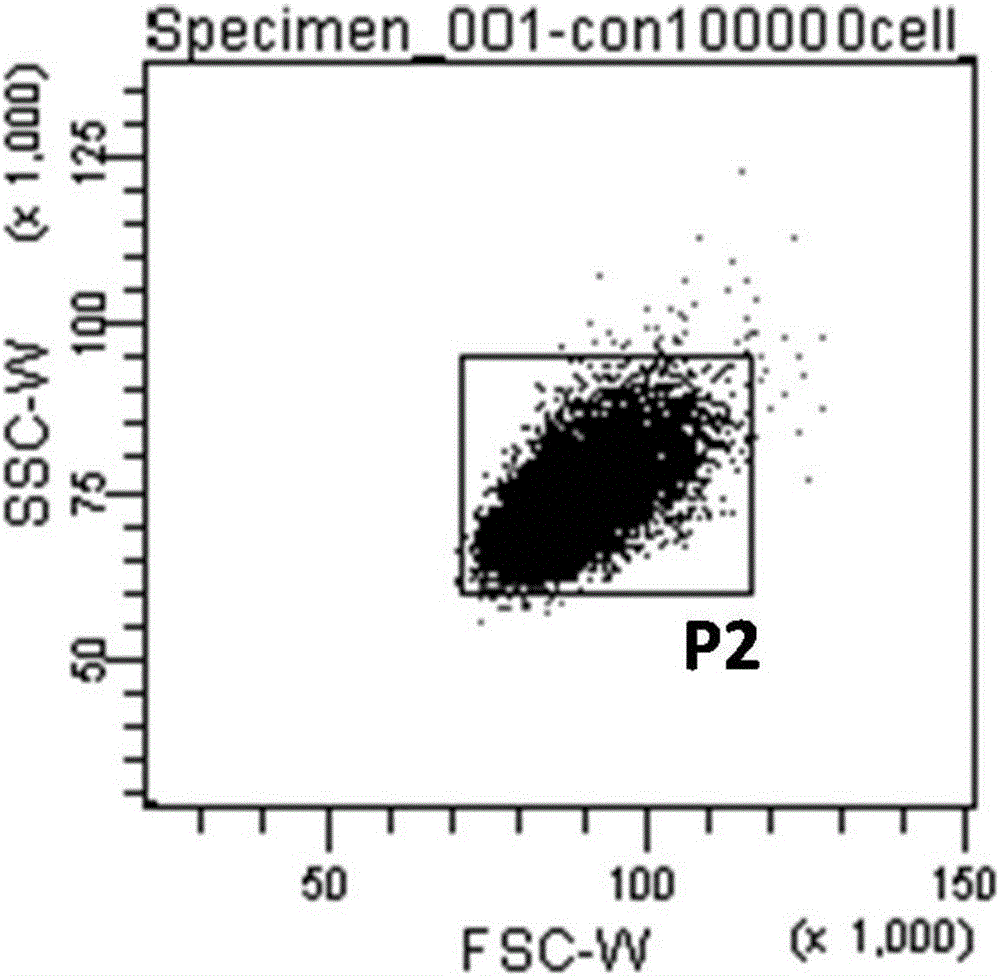
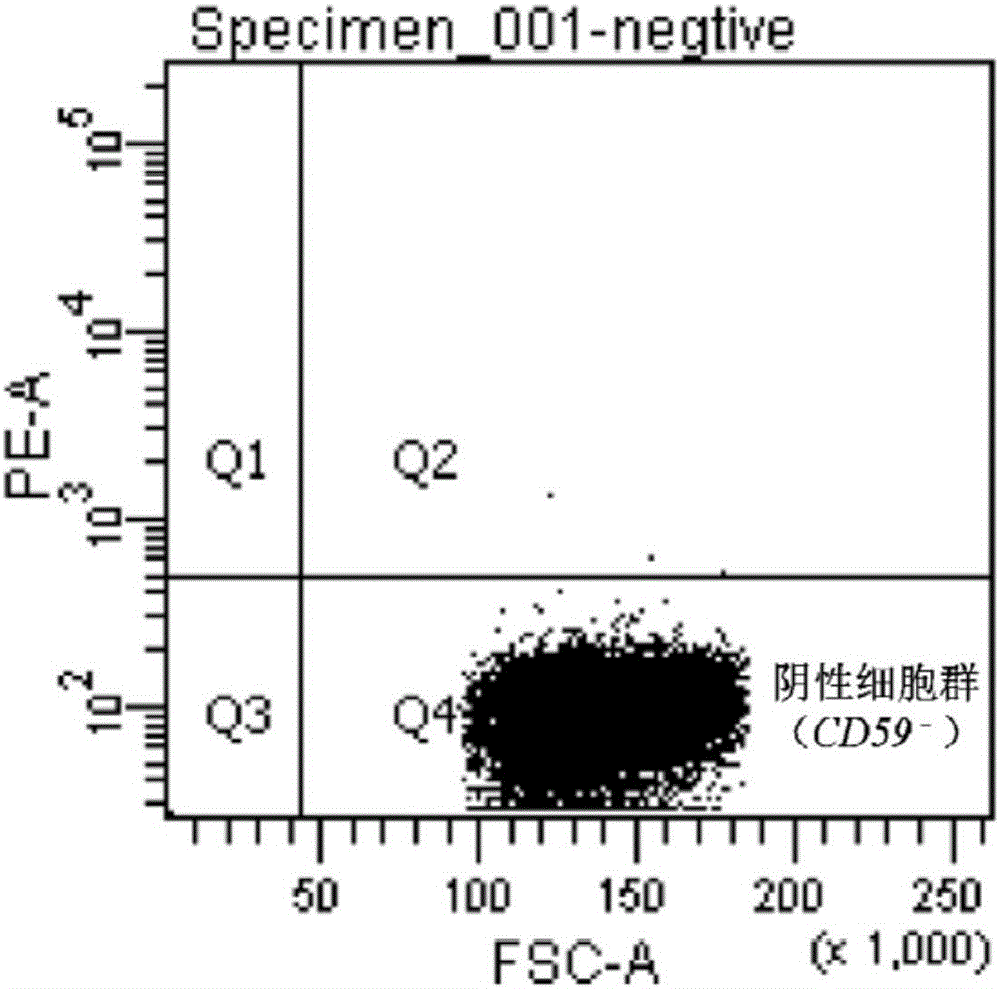
Method for performing high-throughput screening on gene deletion mutation
InactiveCN106834271AHigh sensitivityReduce sensitivityMutant preparationHigh-Throughput Screening MethodsCell processing
The invention discloses a method for performing high-throughput screening on gene deletion mutation. The method comprises the following steps: performing cell treatment and continuous cultivation, collecting cells, binding with fluorescent antibodies, preparing upper sample suspension, and performing cell sorting, cell proliferation and atlas analysis. The method has the following advantages: (1) the method is simple in operation, the whole process of collecting the cells, binding with specific fluorescent protein and sorting only needs 3 to 4 hours, and the detection and screening efficiency of the CD59 gene deletion mutation is improved; and (2) CD59-phenotype single cells with different mutation types and capable of being continuously cultivated can be obtained quickly, the experiment sensitivity and accuracy of a mutation analysis system are effectively improved, and high-throughput screening characteristic is embodied.
Owner:HEFEI INSTITUTES OF PHYSICAL SCIENCE - CHINESE ACAD OF SCI
Bim (Bcl-2 interacting mediator of cell death) gene deletion fluorescent quantitative PCR (polymerase chain reaction) detection primer, probe and detection reagent kit
PendingCN105603069AHigh detection sensitivityEfficient detectionMicrobiological testing/measurementDNA/RNA fragmentationForward primerFluoProbes
The invention discloses a Bim (Bcl-2 interacting mediator of cell death) gene deletion fluorescent quantitative PCR (polymerase chain reaction) detection primer, a probe and a detection reagent kit, and belongs to the field of molecular biological detection. The Bim gene deletion fluorescent quantitative PCR detection primer and the probe comprise forward primers 5'-CAACAAACCCATCAGAACAGACAC-3', reverse primers 5'-ACAGCCTCTATGGAGAACAGTGATT-3 and fluorescent probes 5'-FAM-CAGACACTGGAACAAA-MGB-3'. The detection reagent kit comprises the Bim gene deletion fluorescent quantitative PCR detection primer, the probe, premixed liquid of PCR liquid and positive control samples A. The Bim gene deletion fluorescent quantitative PCR detection primer, the probe and the detection reagent kit have the advantages that Bim gene deletion mutation can be detected by the aid of the Bim gene deletion fluorescent quantitative PCR detection primer, the probe and the detection reagent kit, and the Bim gene deletion fluorescent quantitative PCR detection primer, the probe and the detection reagent kit are simple, convenient and feasible and are high in accuracy and sensitivity and particularly suitable for detecting clinical samples; Bim deletion mutation can be detected, and accordingly the Bim gene deletion fluorescent quantitative PCR detection primer, the probe and the detection reagent kit can bring convenience for guiding individual treatment on bodies.
Owner:ANHUI DAJIAN MEDICAL TECH CO LTD
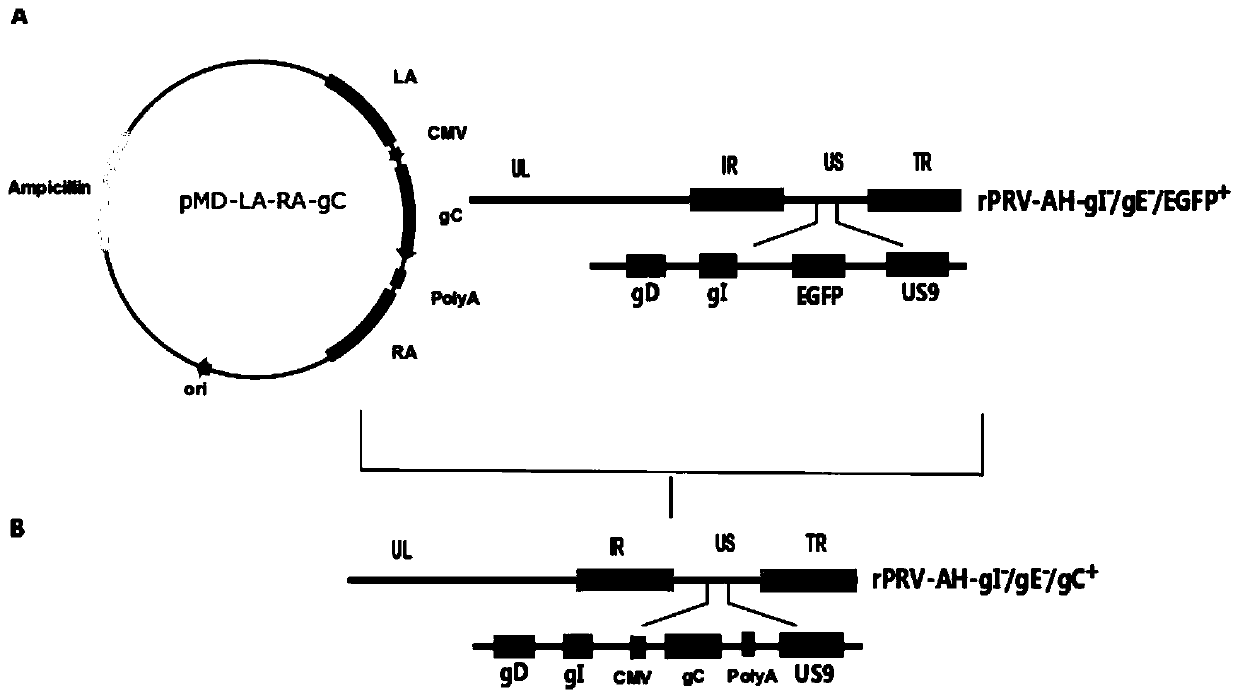
Pseudorabies virus gE/gI deletion mutant strain of double expression gC gene and construction and application thereof
PendingCN109750007AGood immune effectImproving immunogenicityStable introduction of DNAMicroorganism based processesNucleotideFhit gene
The present invention discloses a pseudorabies virus gE / gI deletion mutant strain rPRV-AH-gI- / gE- / gC+ of a double expression gC gene. The pseudorabies virus gE / gI deletion mutant strain is positionedat the position of missing gE / gI gene of recombinant pseudorabies virus rPRV-AH-gI- / gE and is constructed by inserting the gC gene; the nucleotide sequence of the gI / gE gene is shown as SEQ ID NO.1, and the nucleotide sequence of the gC gene is shown as SEQ ID NO.2. Compared with other PRV gene deletion mutant strains, the recombinant pseudorabies virus rPRV-AH-gI / gE- / gC+ inserts the gC gene innovatively by taking the gC gene as one of the main immunogenic proteins of PRV on the basis of the deletion of the gI / gE gene, and induces a body to produce a neutralizing antibody and a cellular immuneresponse. The invention further provides rPRV-AH-gI- / gE- / gC+ inactivated vaccine. The inactivated vaccine has better safety performance, and has better immunogenicity, higher neutralizing antibody level and better immune protection effect compared with an existing PRV mutant strain, can distinguish wild virus infected animals from vaccine immunized animals by an existing PRV gE differentiation diagnosis method, and is expected to be used as a genetic engineering inactivated vaccine for preventing and treating novel epidemic strains of porcine pseudorabies virus.
Owner:SOUTH CHINA AGRI UNIV
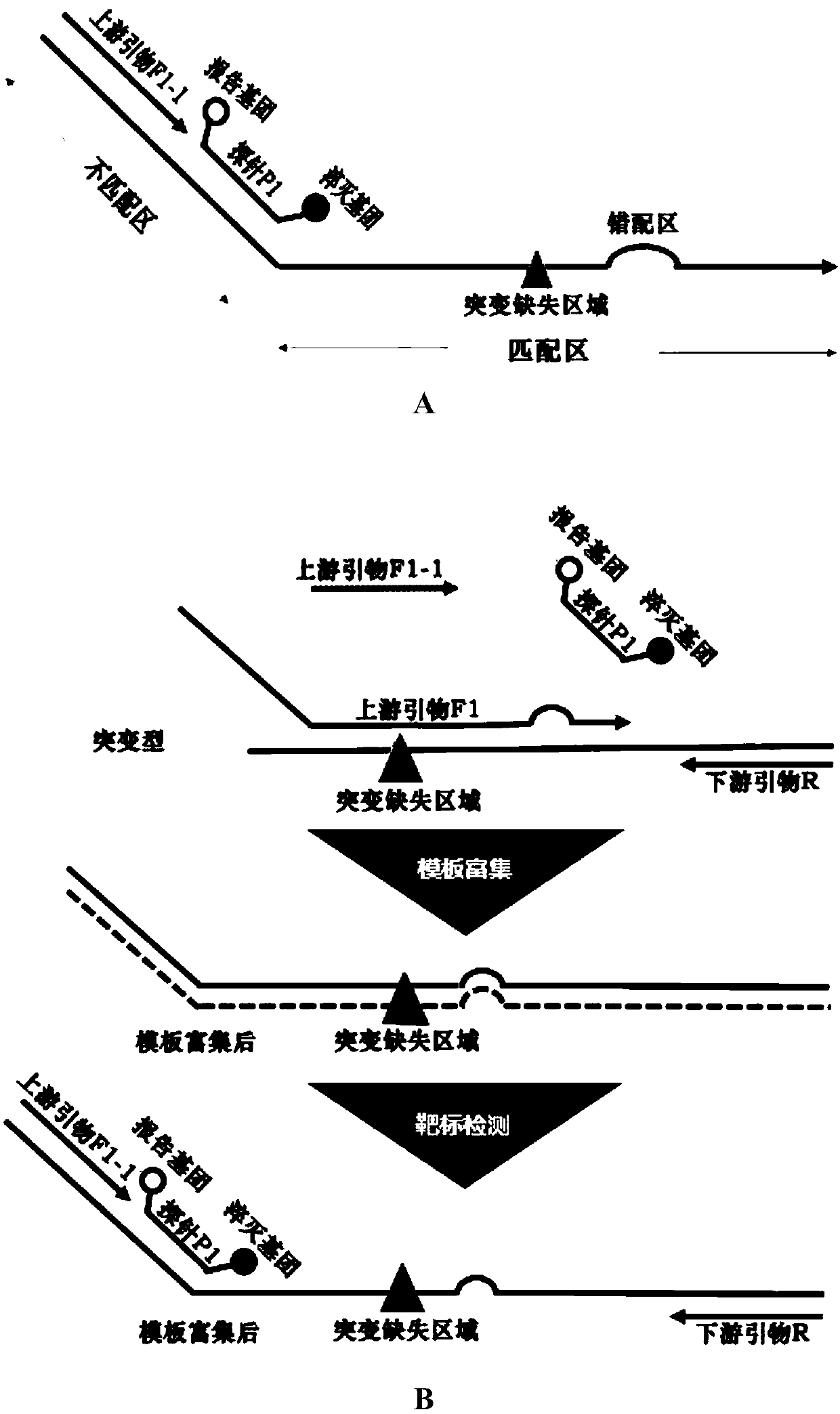
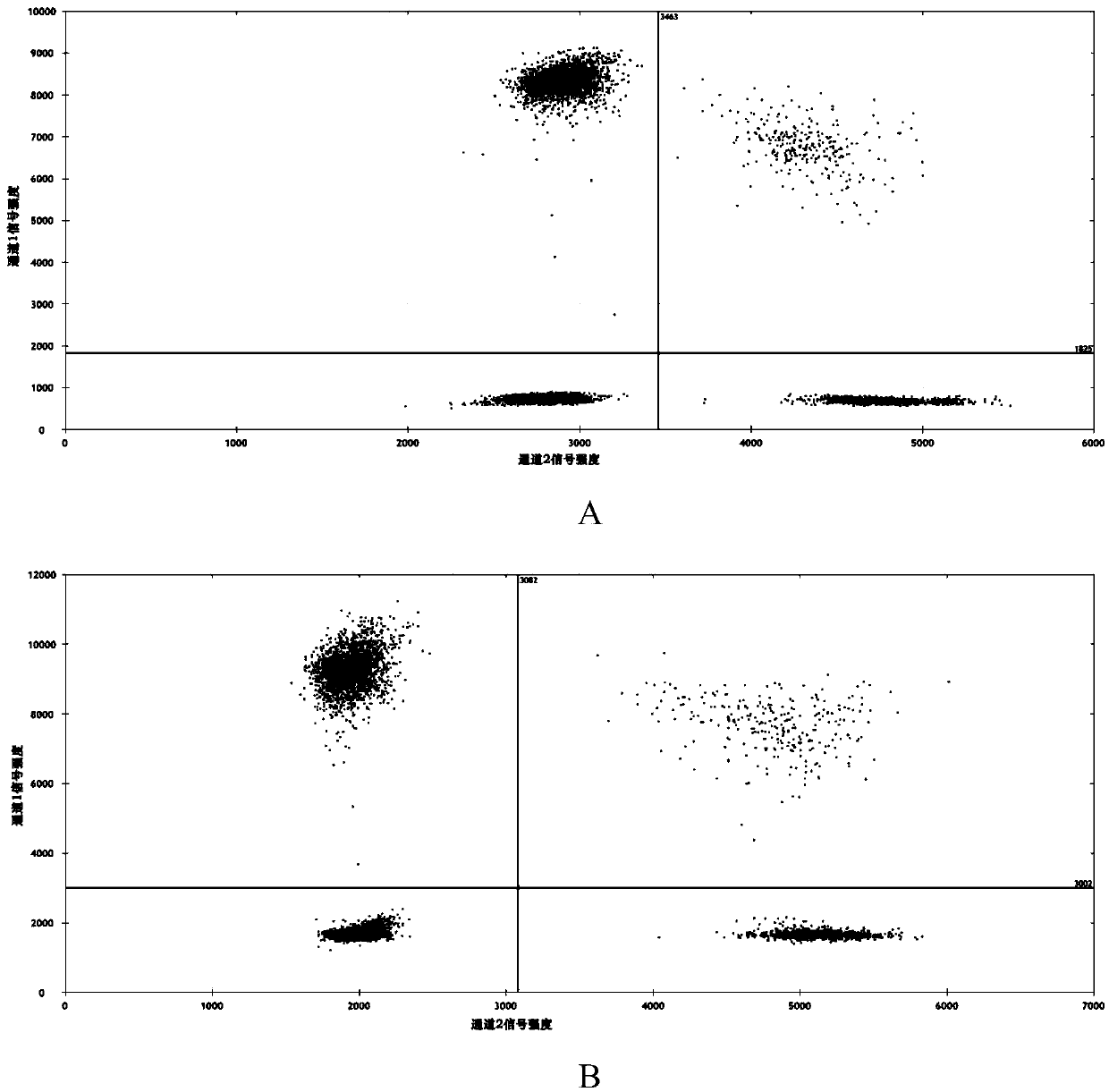
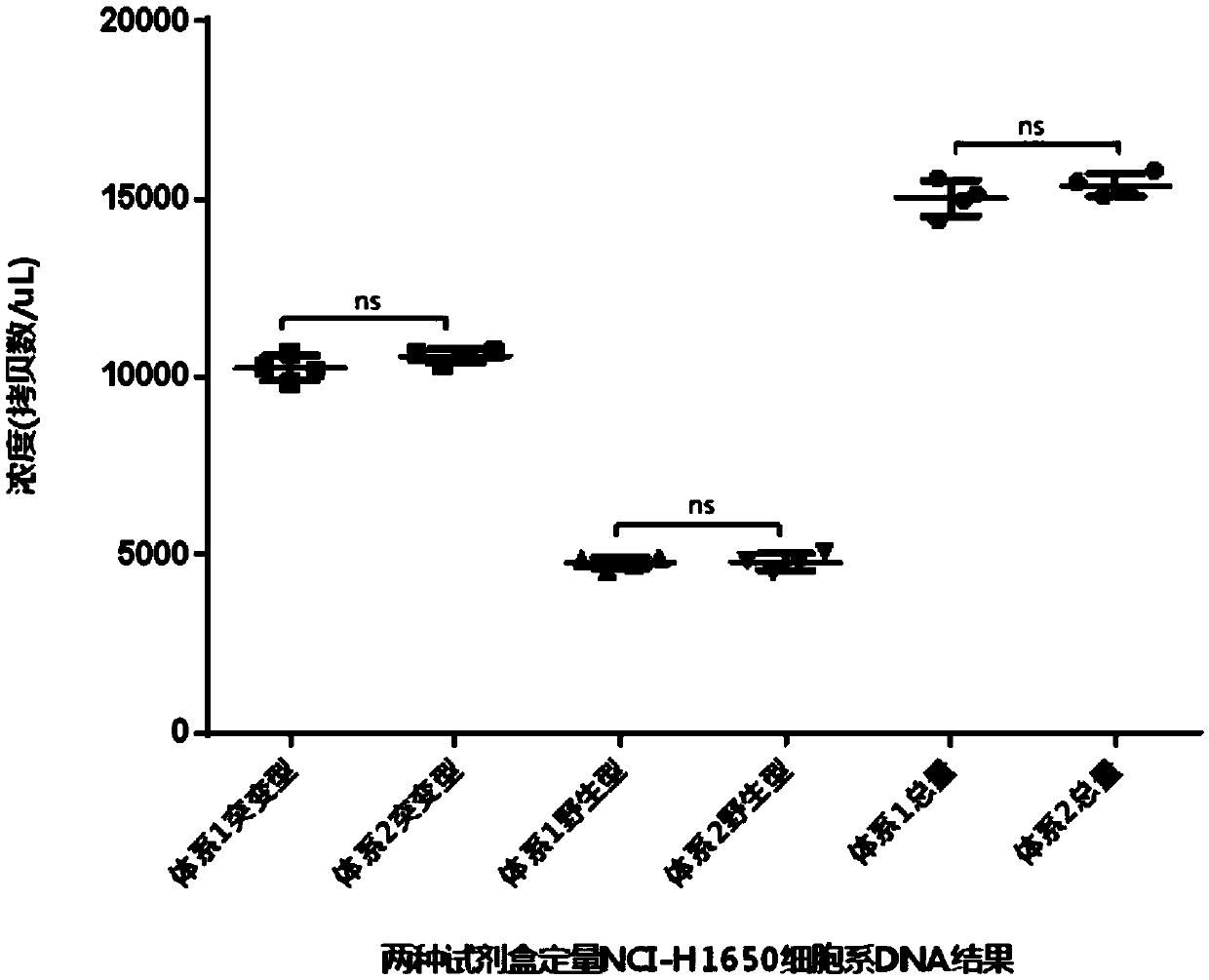
Composition and kit for detecting EGFR gene deletion mutation, and detection method
PendingCN111349691AShorten the lengthHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationWild typeHydrolysis
The invention discloses a composition and a kit for detecting EGFR gene deletion mutation, and a detection method. The composition comprises a mutant-type detection composition and / or a wild-type detection composition, wherein the mutant-type detection composition comprises an upstream primer F1, an upstream primer F1-1, a hydrolysis probe P1 and a downstream primer; the wild-type detection composition comprises an upstream primer F2, an upstream primer F2-1, a hydrolysis probe P2 and a downstream primer, wherein each of the upstream primer F1 and the upstream primer F2 comprises a non-matching region and a matching region; and the matching regions of the upstream primers F1 and F2 are specifically bound to the to-be-detected sequence regions respectively. The unmatched regions of the upstream primers F1 and F2 have the same sequences of the upstream primers and hydrolysis probes for corresponding detection. Compared with the prior art, the composition has the advantages that the sensitivity and the height specificity are remarkably improved, the primers and the test cost are greatly reduced, and the application value is extremely high.
Owner:SICHUAN MACCURA BIOTECH CO LTD
Method and kit for detecting deletion mutation of cell apoptosis regulator gene (BIM)
The invention belongs to the fields of molecular biology and medicine and relates to a method for detecting deletion mutation of a cell apoptosis regulator gene (BIM) and a detection kit applying the method. The method for detecting deletion mutation of the cell apoptosis regulator gene (BIM) comprises a step of detecting deletion of 2903bp on an intron between a third exon and a fourth exon on the BIM gene. The invention also discloses a corresponding detection kit which also contains a primer used for amplifying a commonly deleted region of 2903bp on the BIM. When the method and kit provided by the invention are utilized for detecting BIM deletion condition, operation is simple and easy, rapidness and high efficiency are realized, and cost is low.
Owner:SHANGHAI CHEST HOSPITAL
Sphingomonas paucimobilis gene knockout mutant strain and construction method thereof
InactiveCN107384843AInhibitionHigh purityBacteriaOxidoreductasesSmeryngolaphria numitorMutant strain
The invention provides a sphingomonas paucimobilis gene knockout mutant strain and a construction method thereof. The strain is collected in China Center for Type Culture Collection and has the collection number of CCTCC No. 2016610. A PHB deletion sphingomonas paucimobilis gene knockout mutant strain is constructed, and genes of acetoacetyl coenzyme A reductase in a PHB synthetic route of sphingomonas paucimobilis are knocked out by utilizing homologous recombination so as to obtain the gene deletion mutant strain, so that production of the PHB is inhibited, and the purity and transparency of colloid are improved; and meanwhile, the colloid productive rate and colloid production stability are maintained, so that the possibility of removing transparent high acyl gellan gum of the PHB in large-scale production is greatly improved.
Owner:上海北连生物科技有限公司
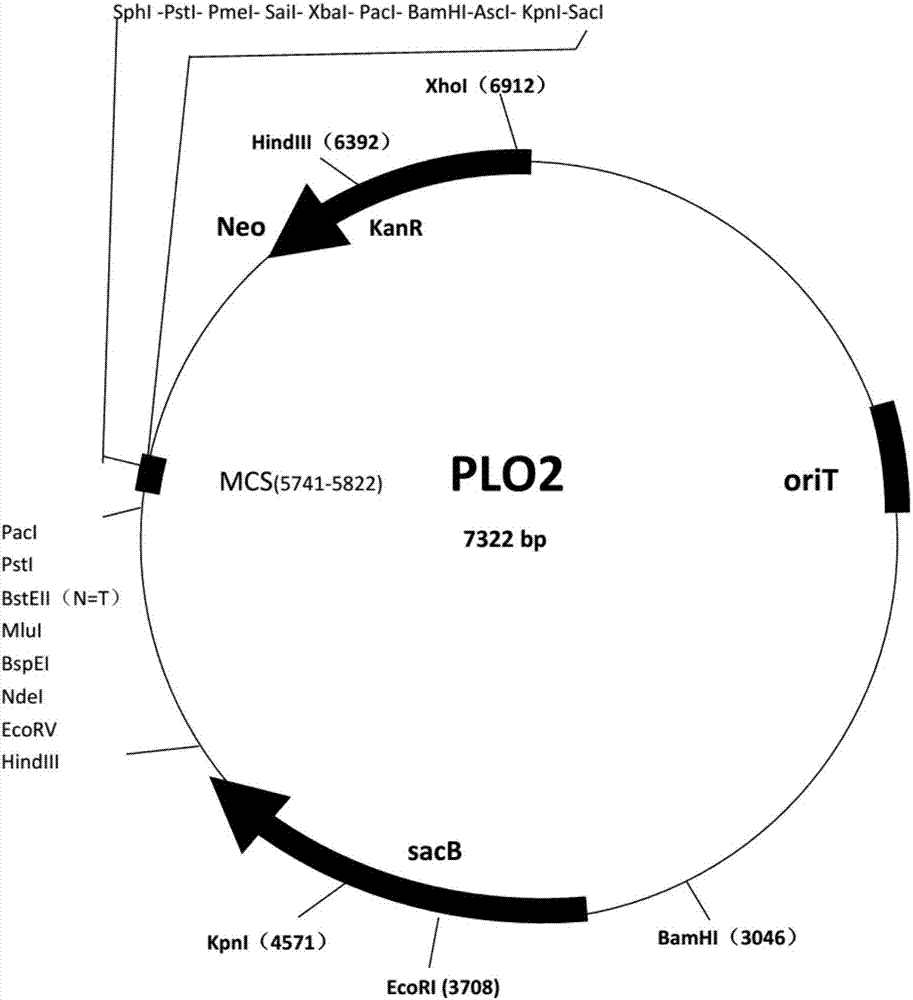
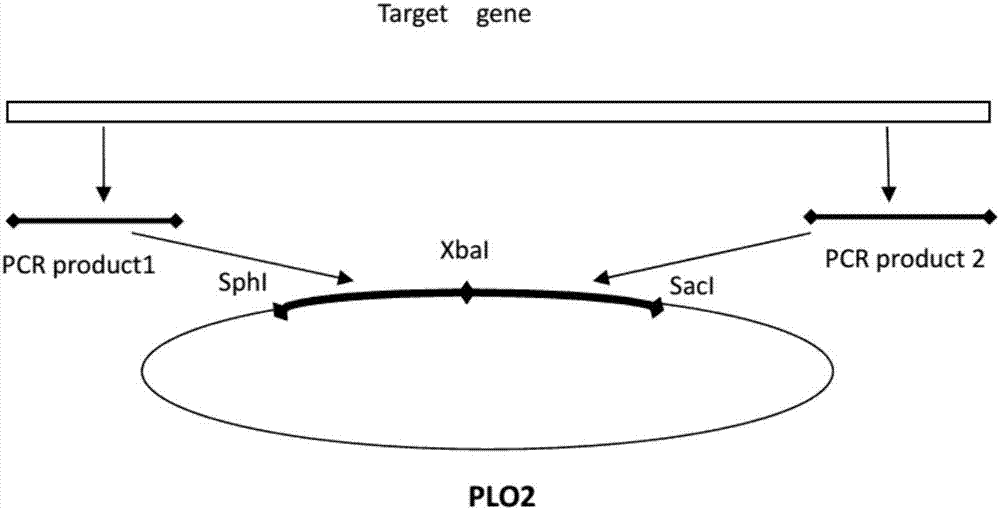
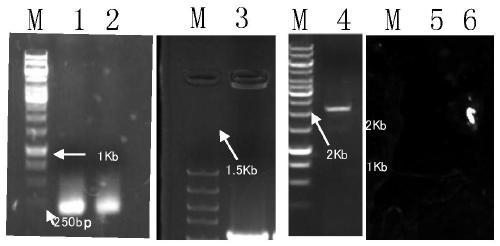
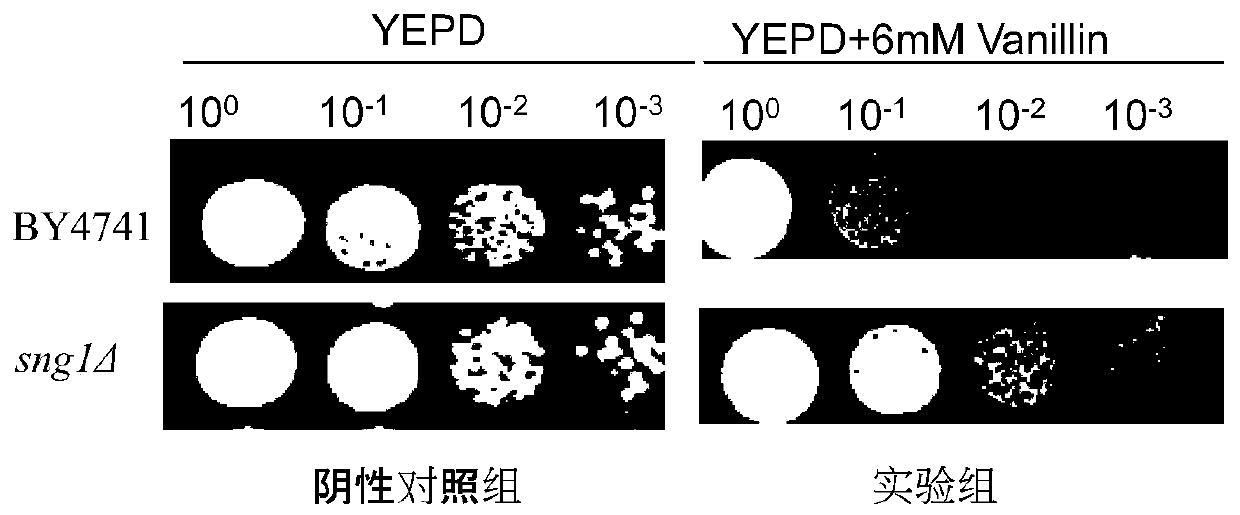
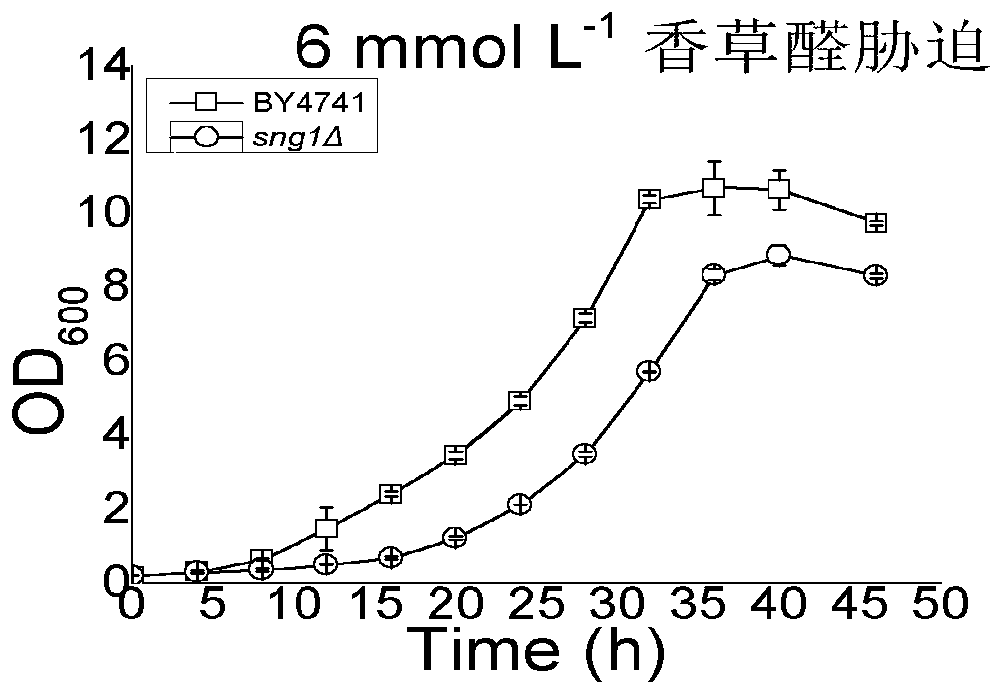
Application of gene SNG1 deletion in improving vanillic aldehyde resistance of saccharomyces cerevisiae
ActiveCN111549073AIncrease resistanceShorten the lag periodFungiStable introduction of DNACelluloseNucleotide
The invention discloses an application of saccharomyces cerevisiae SNG1 gene deletion in improving vanillic aldehyde resistance. The gene sequence of the related SNG1 gene deletion mutant is composedof -18 to +203 bp nucleotide fragments of a saccharomyces cerevisiae SNG1 gene, nucleotide fragments of loxp-KanMX4-loxp and +1446 to +1644 bp nucleotide fragments of the saccharomyces cerevisiae SNG1gene, and the nucleotide sequence of the mutant is as shown in SEQ ID No.1. Experiments prove that under no stress, the wild type is not different from the growth of the sng1 delta mutant disclosed by the invention; however, under the stress of vanillic aldehyde, the maximum specific growth rate of the sng1 delta mutant is 70% higher than that of a control strain, and the specific consumption rate of vanillic aldehyde is 52% higher than that of vanillic aldehyde. It is prompted that the mutant strain is suitable for vanillin / alcohol production or construction of a high-vanillin-tolerance saccharomyces cerevisiae strain for producing second-generation fuel ethanol or other high-value compounds by taking lignocellulose as a raw material.
Owner:QILU UNIV OF TECH
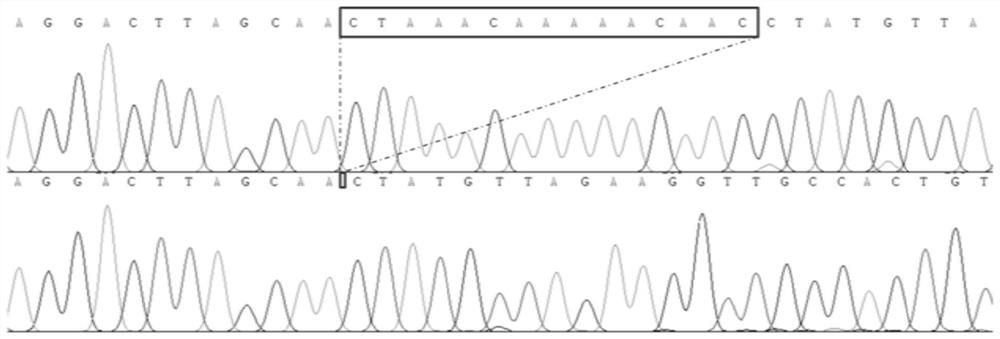
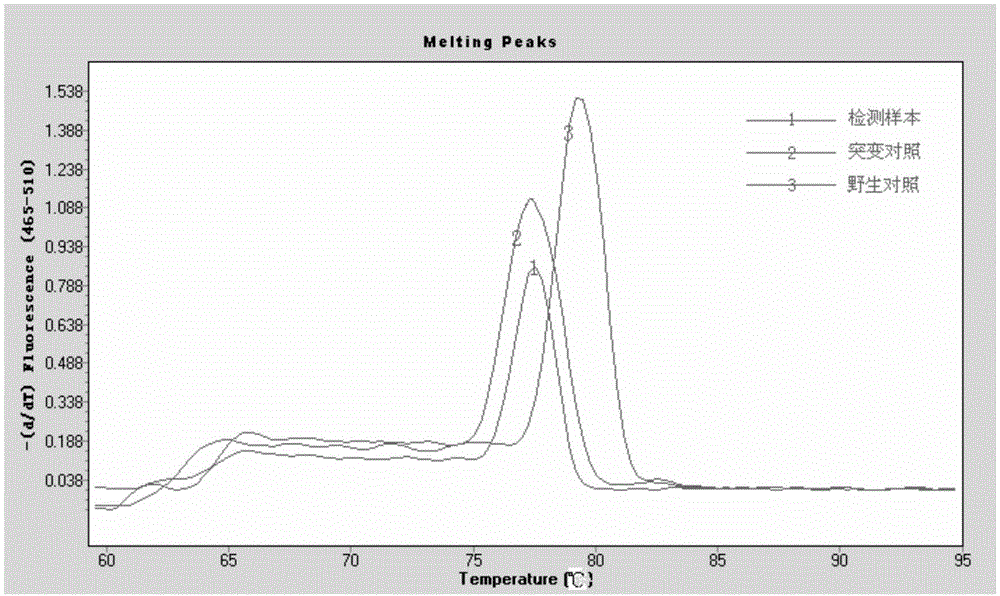
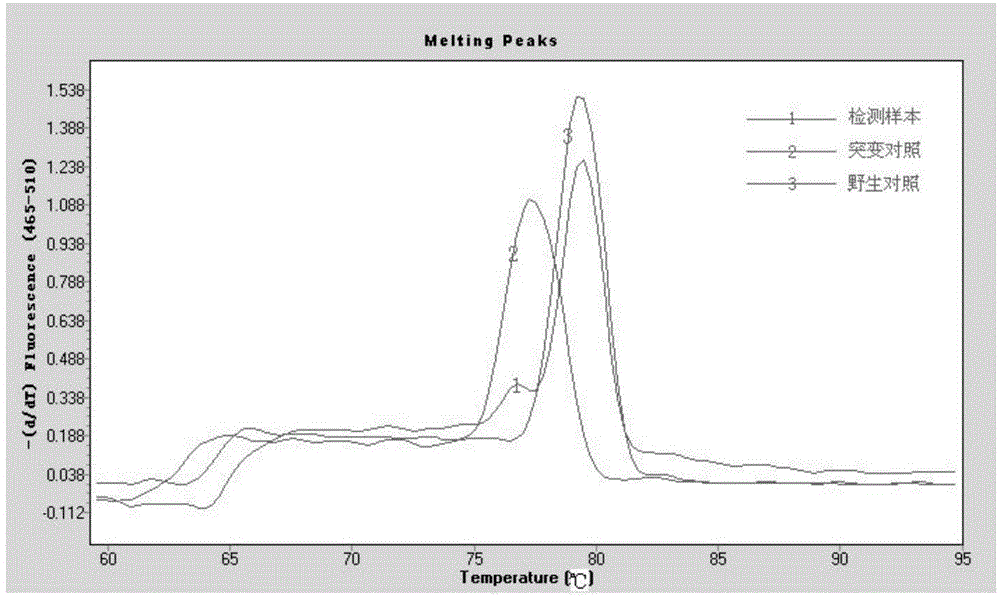
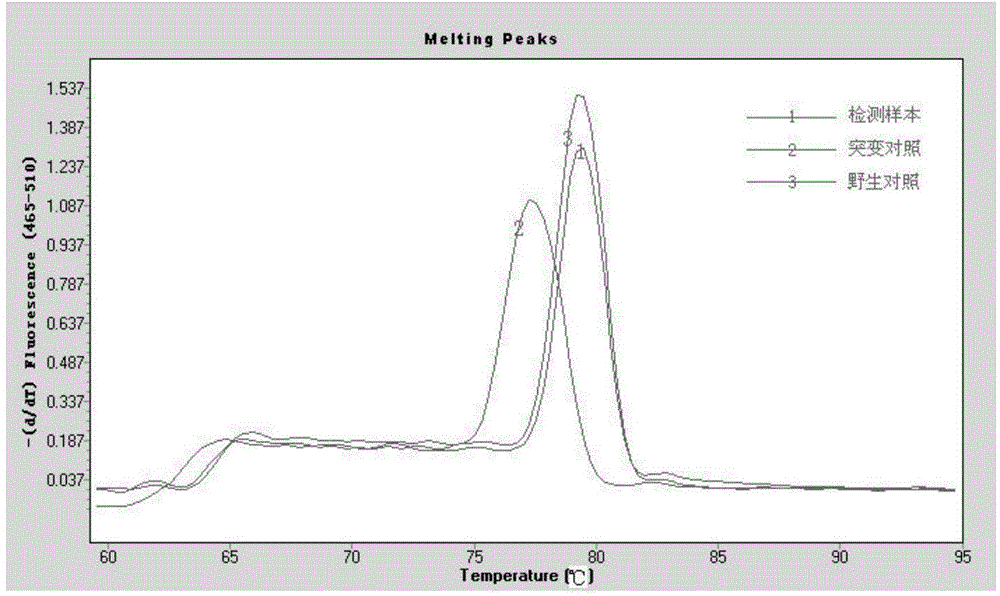
Botrytis cinerea mutant strain [delta]bcscd1 capable of producing scytalone and construction method thereof
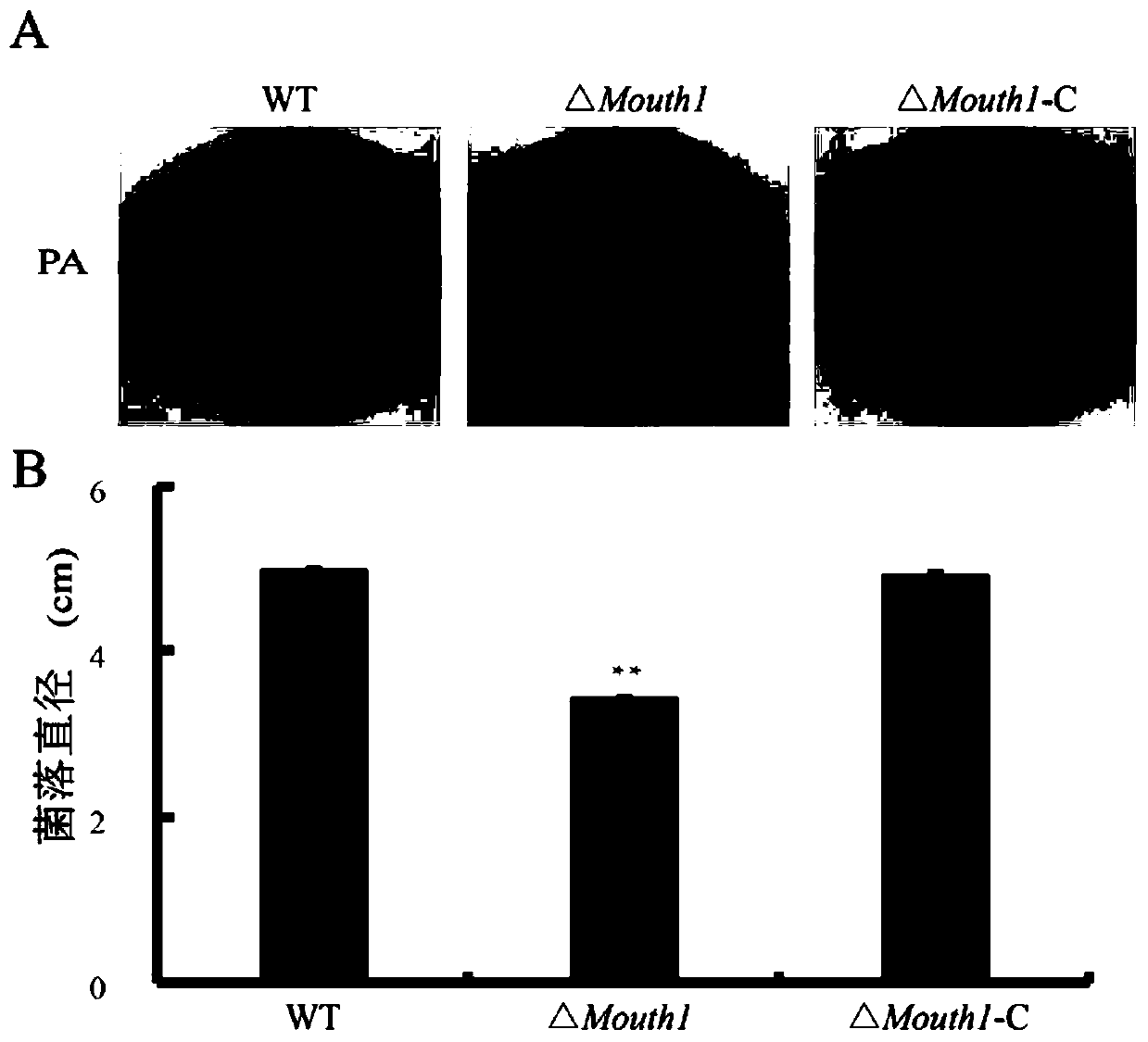
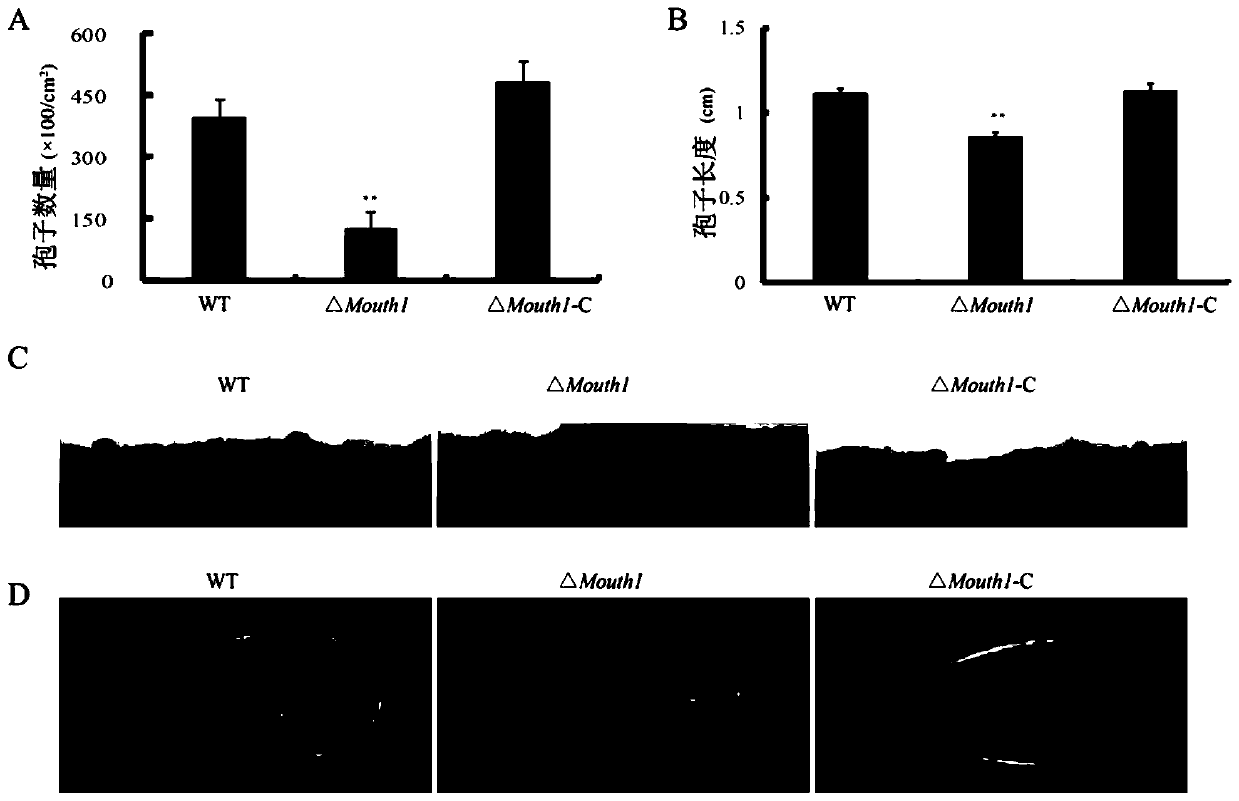
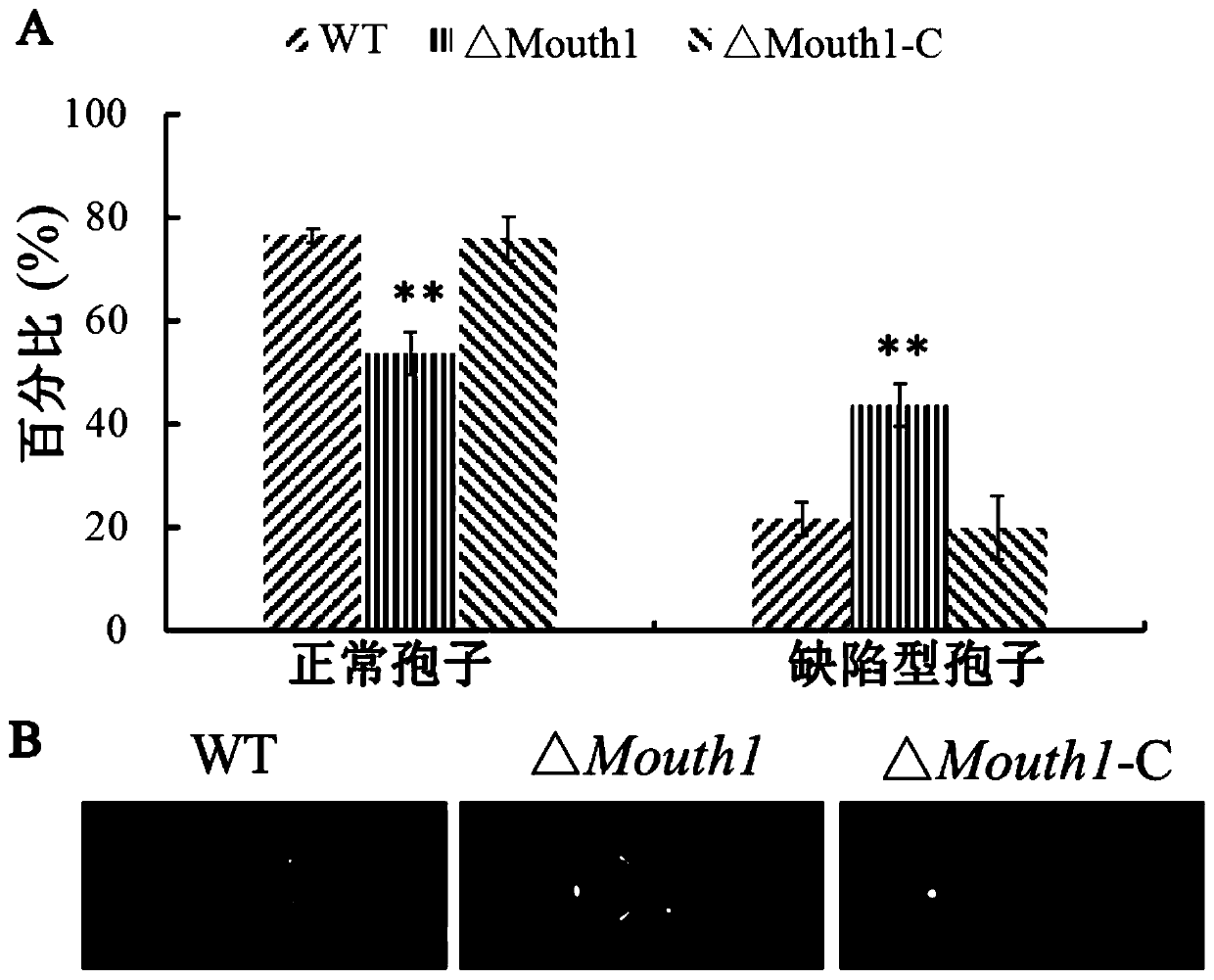
The invention belongs to the field of biological technology. The wild-type cinerea cinerea strain is used as the background strain to knock out the gene of a key enzyme cylindroxone dehydratase in the melanin synthesis pathway, and construct a gene deletion mutation of the cinerea cinerea capable of producing a large amount of cylindroxone The main steps of the strain Δbcscd1 are as follows: use fusion PCR technology to fuse the 5'-UTR and 3'-UTR of the target gene bcscd1 with the upstream and downstream coding regions of the hygromycin fragment Hph to obtain the upstream and downstream fusion fragments, and use PEG-mediated The protoplast transformation method was used to transform the fusion fragment into wild-type protoplasts for double-crossover homologous recombination, and the gene deletion mutant strain Δbcscd1 was obtained through screening and identification. The gene deletion mutant strain constructed by the method of the invention has high genetic stability and low toxicity. The present invention also proposes a method for specifically purifying cylindroporin through steps such as extraction, rotary steaming and column chromatography.
Owner:EAST CHINA NORMAL UNIV
Pyricularia grisea mitophagy related pathogenic factor, gene and application thereof
ActiveCN110669115ALow sporulationReduce pathogenicityMicrobiological testing/measurementDepsipeptidesBiotechnologyAntifungal drug
The invention discloses a pyricularia grisea mitophagy related pathogenic factor, a gene and an application thereof. The gene deletion mutation caused by homologous recombination causes slow growth ofpyricularia grisea, reduction of sporulation quantity, reduction of formation of infected hyphae and great reduction of pathogenicity on susceptible rice varieties. Therefore, the most important purpose of the pathogenic factor is to design and screen a compound capable of destroying the expression and shearing of the gene and the expression of the encoded protein, or design and screen a compoundcapable of modifying an amino acid sequence of the protein by applying the achievements, thereby developing a new antifungal drug. Meanwhile, the gene family where the gene is located does not have ahomologous gene in a plant, so that an antifungal drug developed by taking the gene as a target has relatively small influence on a host plant.
Owner:CHINA NAT RICE RES INST
Seneca recombinant virus of recombinant A-type foot-and-mouth disease virus VP1 gene, recombinant vaccine strain and preparation method and application of recombinant vaccine strain
ActiveCN111996201AAchieving antigen matchingAchieve immune responsivenessSsRNA viruses positive-senseViral antigen ingredientsAntigenDisease
The invention provides a Seneca recombinant virus of a recombinant A-type foot-and-mouth disease virus VP1 gene, a recombinant vaccine strain and a preparation method and application of the recombinant vaccine strain, and relates to the technical field of gene engineering. The invention provides a Seneca recombinant nucleic acid, the Seneca recombinant virus containing the recombinant nucleic acid, the Seneca recombinant vaccine strain containing the Seneca recombinant virus, and a preparation method and application of the vaccine strain. An SVV / FJ / 001 strain is subjected to gene deletion mutation transformation, the VP1 gene of the A-type FMDV is fused into cDNA of the SVV / FJ / 001 strain to obtain the Seneca recombinant virus, the recombinant virus can express the fused gene, and an expression product has good reactogenicity; and the obtained vaccine strain is high in antigen productivity, the pathogenicity is remarkably reduced, even no pathogenicity is caused to pigs, the immune response of SVA can be stimulated after animals are immunized by the inactivated vaccine, the immunocompetence aiming at fusion genes can be generated, and the vaccine strain can be used for preventing and controlling Seneca virus and one or more non-Seneca virus.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
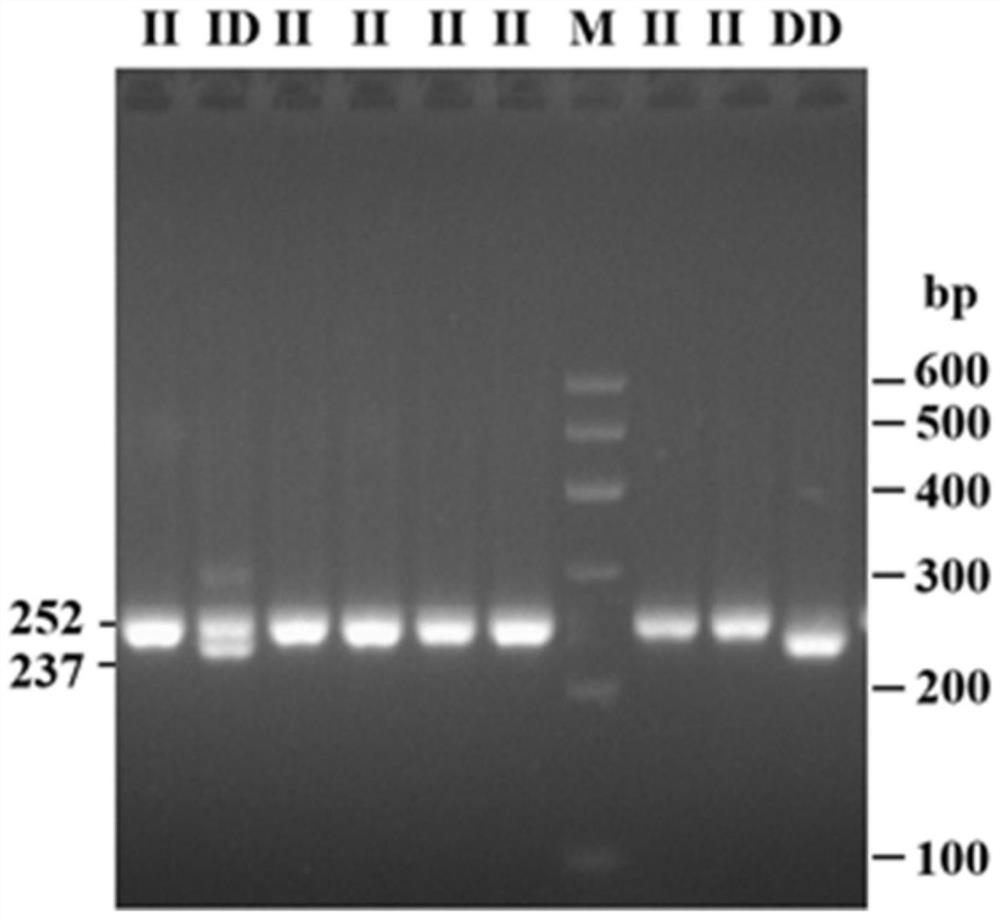
Detection method for bovine FRAS1 gene insertion/deletion mutation and application thereof
ActiveCN112795667AEasy to detectAccurate detectionMicrobiological testing/measurementFRAS1 GENEGenotype
The invention discloses a detection method for bovine FRAS1 gene insertion / deletion mutation and application thereof. Bovine whole genome DNA is used as a template, a part of fragments of the bovine FRAS1 gene are amplified through PCR, and agarose gel electrophoresis is applied to identify the genotype of 15-bp deletion mutation sites of the individual FRAS1 gene. According to a character correlation analysis result, the genotype of the 15-bp deletion mutation site of the FRAS1 gene is remarkably related to the breeding character of the cow. Therefore, the FRAS1 gene 15-bp deletion mutation site can be used for early marker-assisted selection of cow reproductive traits so as to accelerate establishment of cow populations with excellent genetic resources.
Owner:NORTHWEST A & F UNIV
CFTR (Cystic Fibrosis Transmembrane Conductance Regulator) gene deletion mutation form of cystic fibrosis patients and application of CFTR gene deletion mutation form
ActiveCN106674344AMicrobiological testing/measurementGenetic engineeringHuman DNA sequencingNucleotide
The invention discloses a CFTR (Cystic Fibrosis Transmembrane Conductance Regulator) gene deletion mutation form of cystic fibrosis patients and application of the CFTR gene deletion mutation form. The CFTR gene deletion mutation form is used for protecting proteins including: (a1) a protein obtained by deleting a 508th amino acid residue of a CFTR; and (a2) a protein obtained by deleting 476th to 478th amino acid residues of the CFTR. The CFTR gene deletion mutation form is also used for protecting application of substances for detecting mutation A and / or B to preparation of a kit, wherein the mutation A: 128th to 130th nucleotide deletion of a sequence 3 in a human genome; and the mutation B: 34th to 42nd nucleotide deletion of the sequence 3 in the human genome. Functions of the kit comprise: (c1) evaluation of risks of cystic fibrosis of a person to be detected; (c2) evaluation of the risks of the cystic fibrosis of the person to be detected or offspring of parents to be detected; and (c3) diagnosis or auxiliary diagnosis for judging whether the person to be detected is a patient suffering from the cystic fibrosis or not. The CFTR gene deletion mutation form has an important application value on the diagnosis of the patient suffering from the cystic fibrosis.
Owner:BEIJING CHILDRENS HOSPITAL AFFILIATED TO CAPITAL MEDICAL UNIV
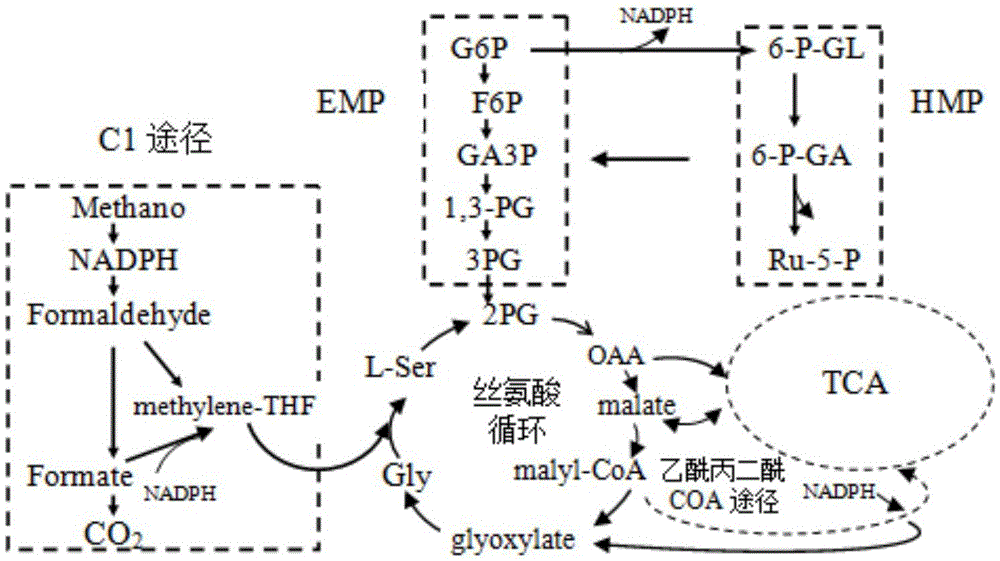
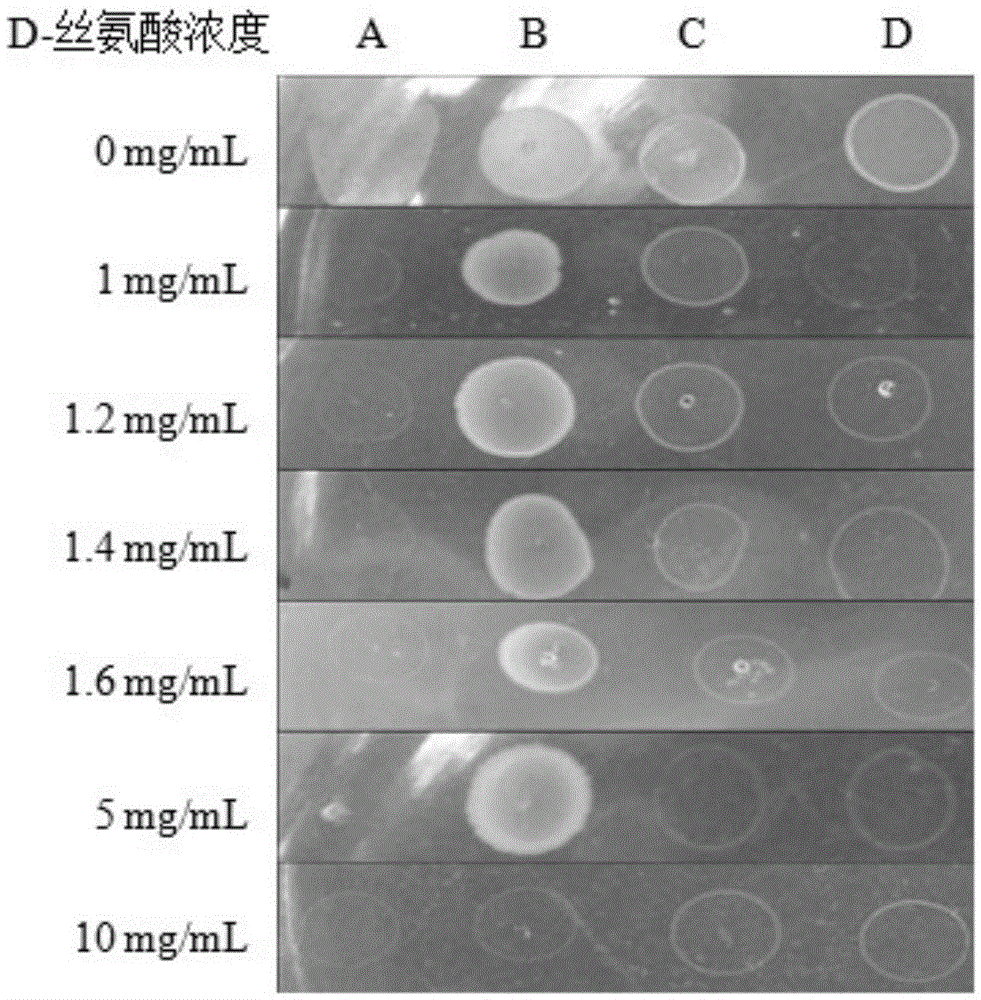
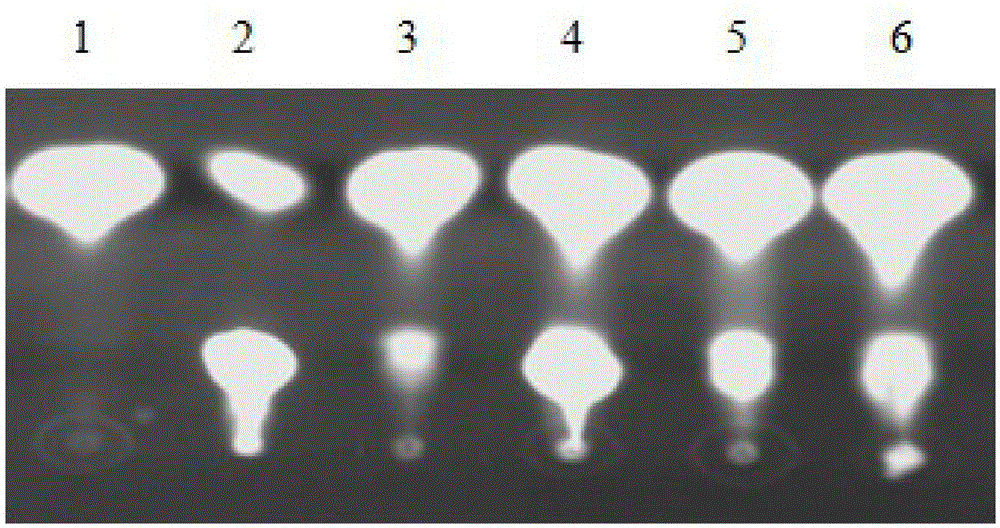
Gene of coded 6-phosphogluconate dehydrogenase and application thereof
ActiveCN105602966AStrong ability to produce L-serineHigh ability to produce L-serineOxidoreductasesFermentationNucleotide6-phosphogluconic acid
The invention provides a gene of coded 6-phosphogluconate dehydrogenase and application thereof. The nucleotide sequence is shown in SEQ ID NO:1. The 6-phosphogluconate dehydrogenase gene pgdhD2 is derived from Methylobacterium sp. MB200 and has the L-serine inhibiting effect. The Methylobacterium sp. MB200 is used as an original strain, pgdh gene deletion mutation DMB is built through a homologous double-crossover method, an L-serine high-yield mutant strain with the L-serine producing capacity greatly higher than that of original strain Methylobacterium sp. MB200 is obtained, and the tolerated D-serine concentration of the L-serine high-yield mutant strain is greatly higher than that of the original strain. The pgdh gene deletion mutation stain has the good application prospect in production of L-serine through a fermentation method.
Owner:GUANGXI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com



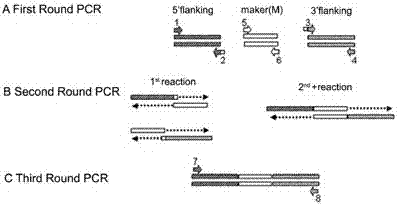
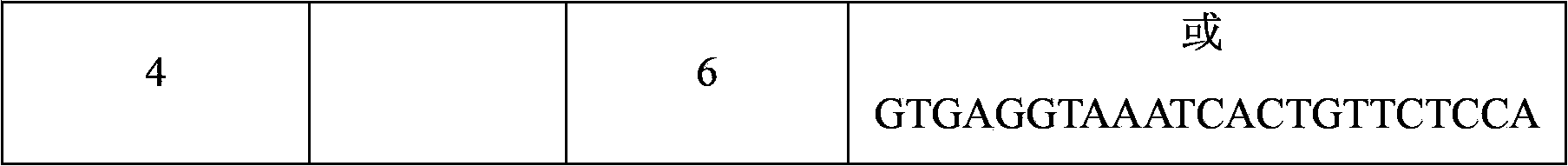
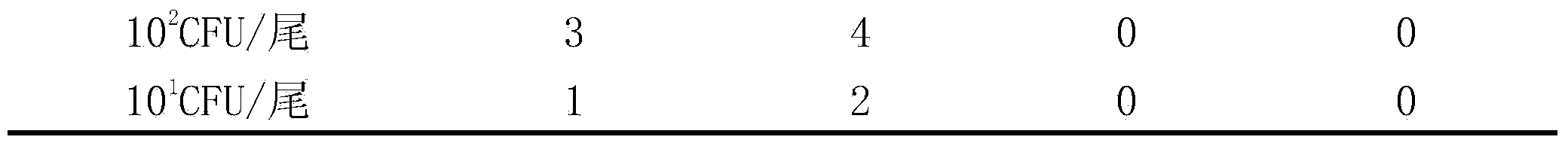


![Botrytis cinerea mutant strain [delta]bcscd1 capable of producing scytalone and construction method thereof Botrytis cinerea mutant strain [delta]bcscd1 capable of producing scytalone and construction method thereof](https://images-eureka.patsnap.com/patent_img/43882c87-f06a-451b-9abe-d48dfc761186/HDA0001358282710000011.png)
![Botrytis cinerea mutant strain [delta]bcscd1 capable of producing scytalone and construction method thereof Botrytis cinerea mutant strain [delta]bcscd1 capable of producing scytalone and construction method thereof](https://images-eureka.patsnap.com/patent_img/43882c87-f06a-451b-9abe-d48dfc761186/HDA0001358282710000012.png)
![Botrytis cinerea mutant strain [delta]bcscd1 capable of producing scytalone and construction method thereof Botrytis cinerea mutant strain [delta]bcscd1 capable of producing scytalone and construction method thereof](https://images-eureka.patsnap.com/patent_img/43882c87-f06a-451b-9abe-d48dfc761186/HDA0001358282710000013.png)