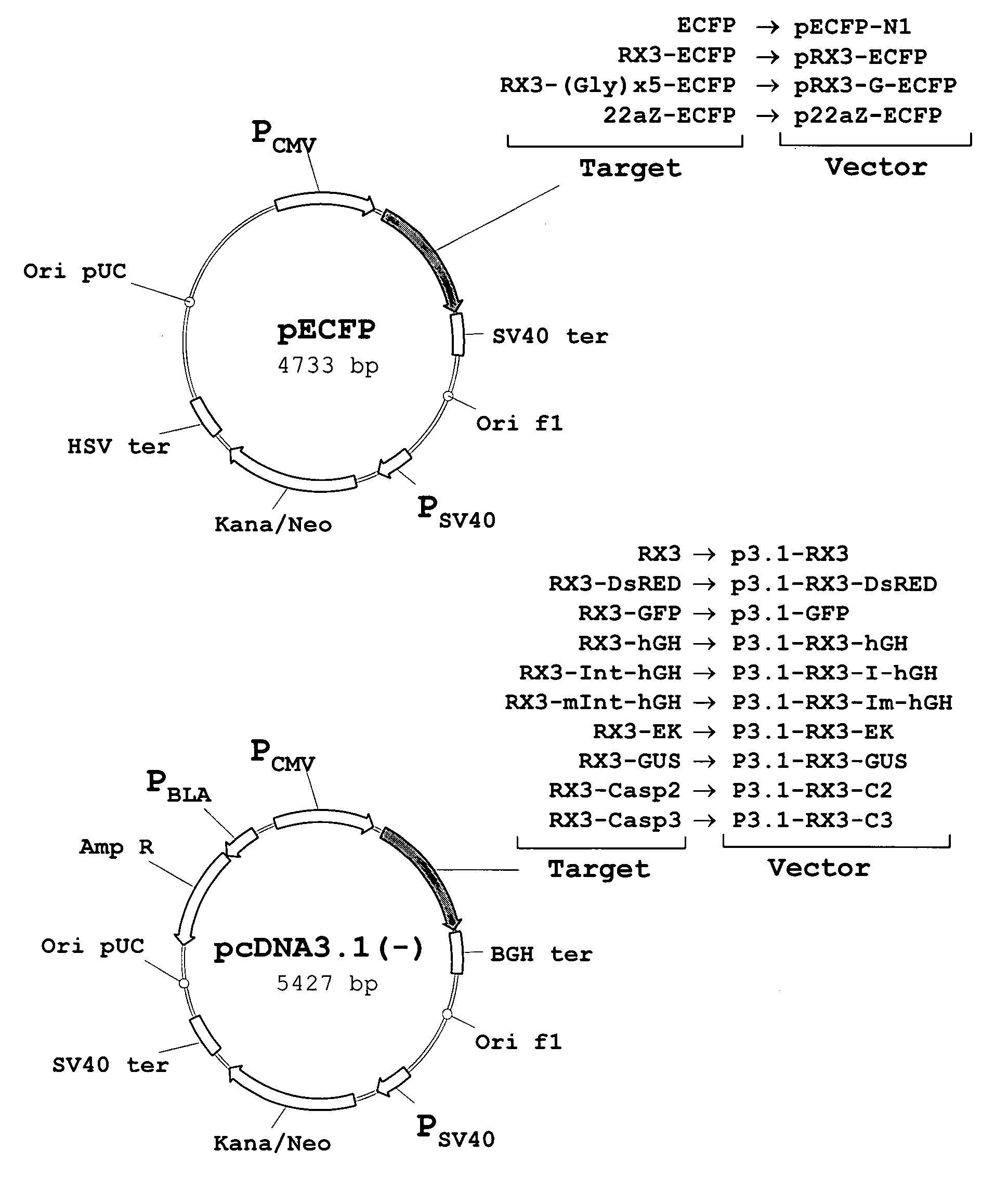
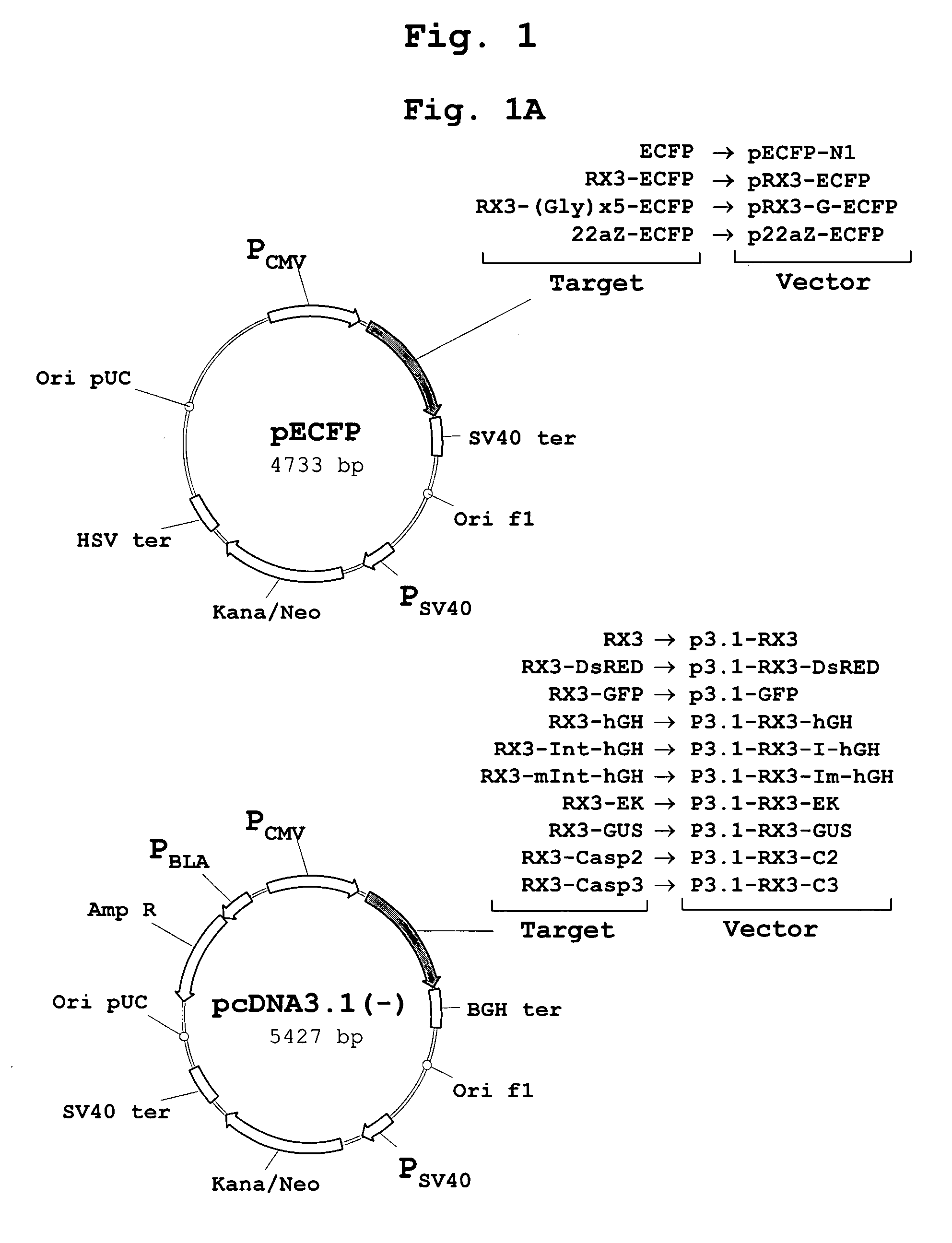
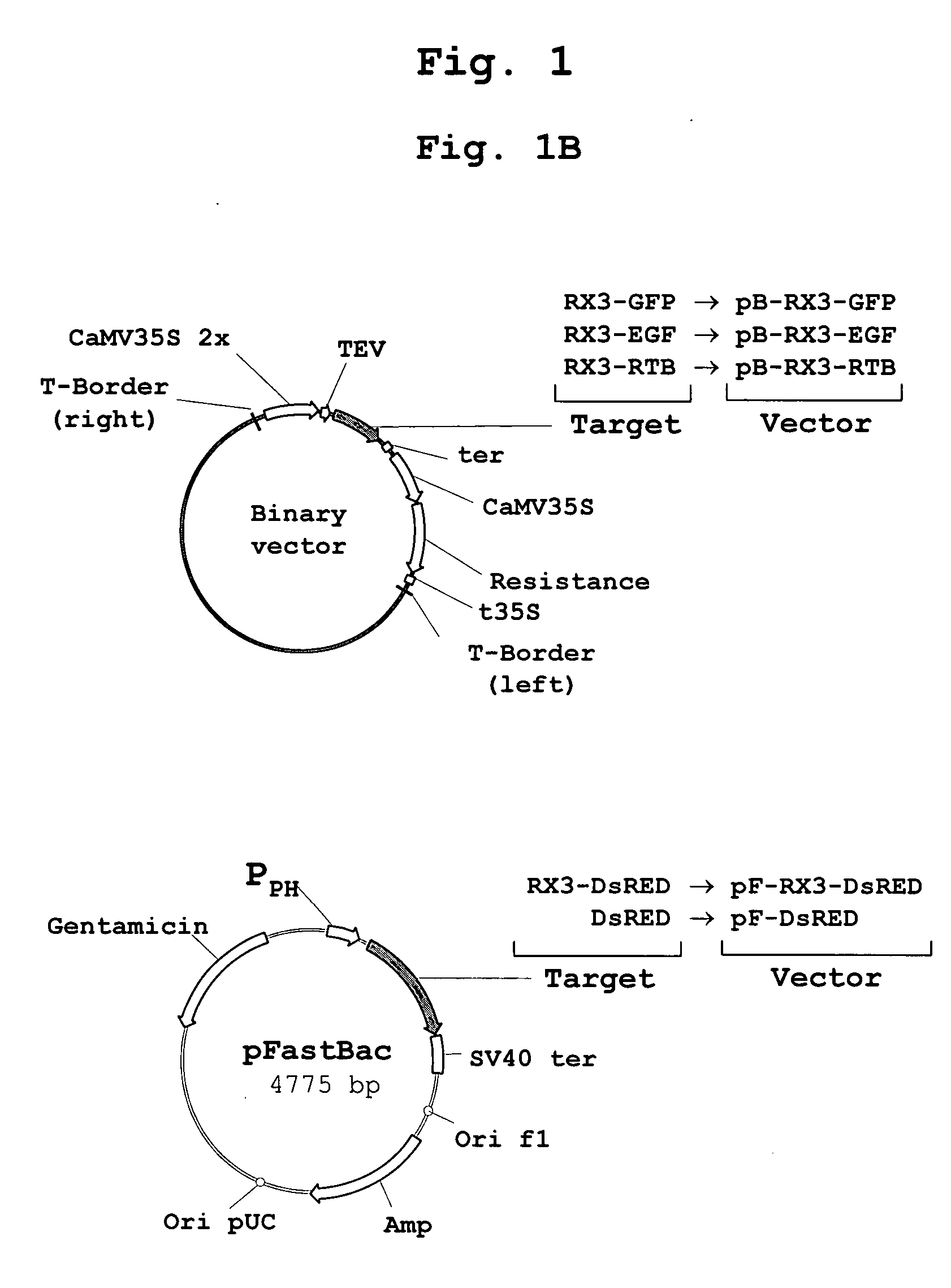
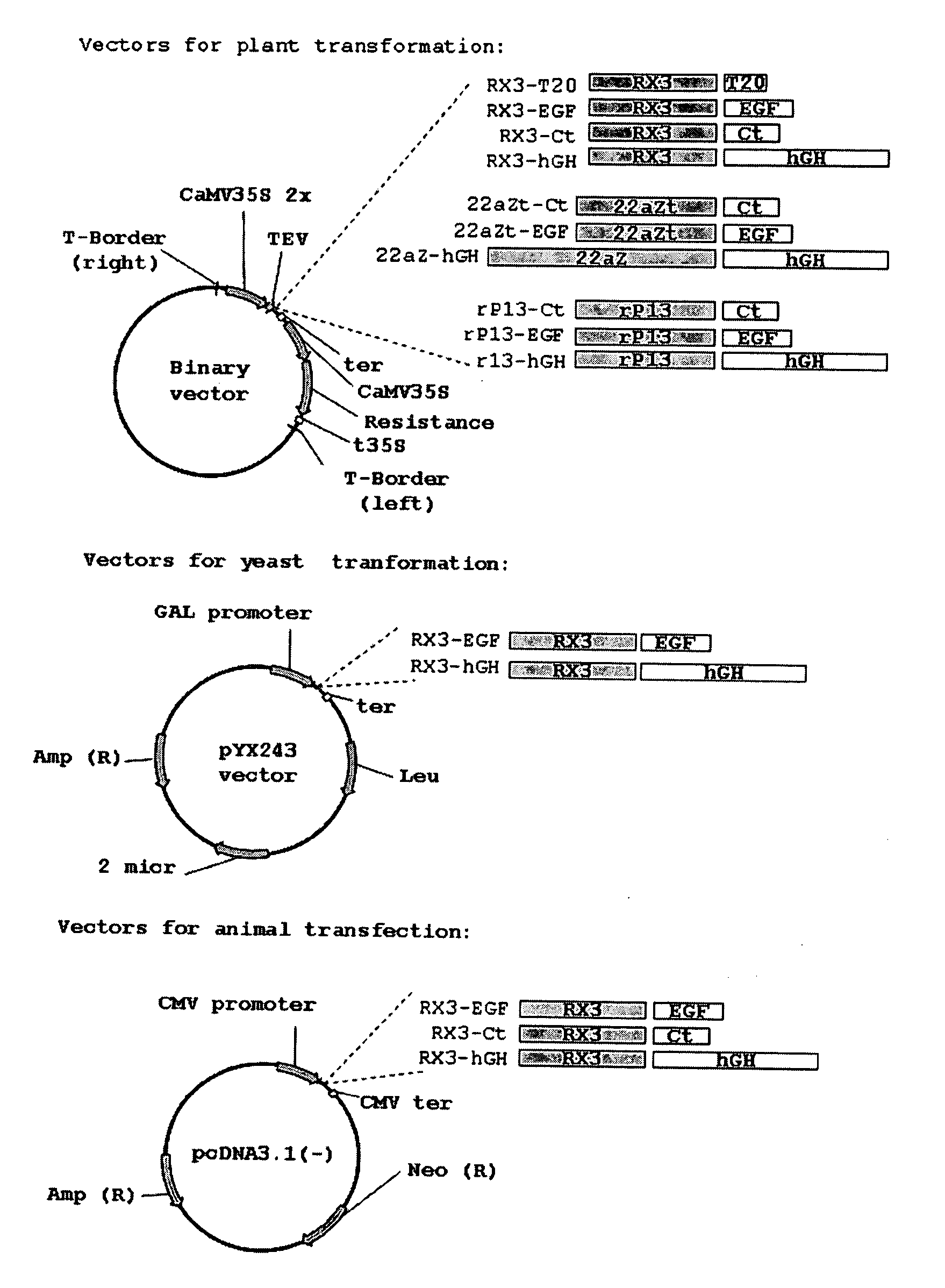
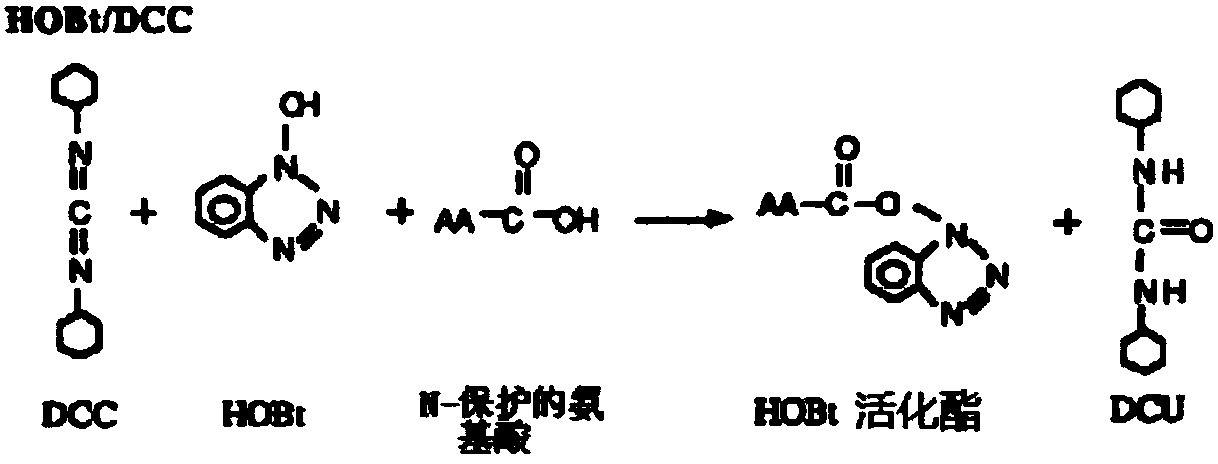
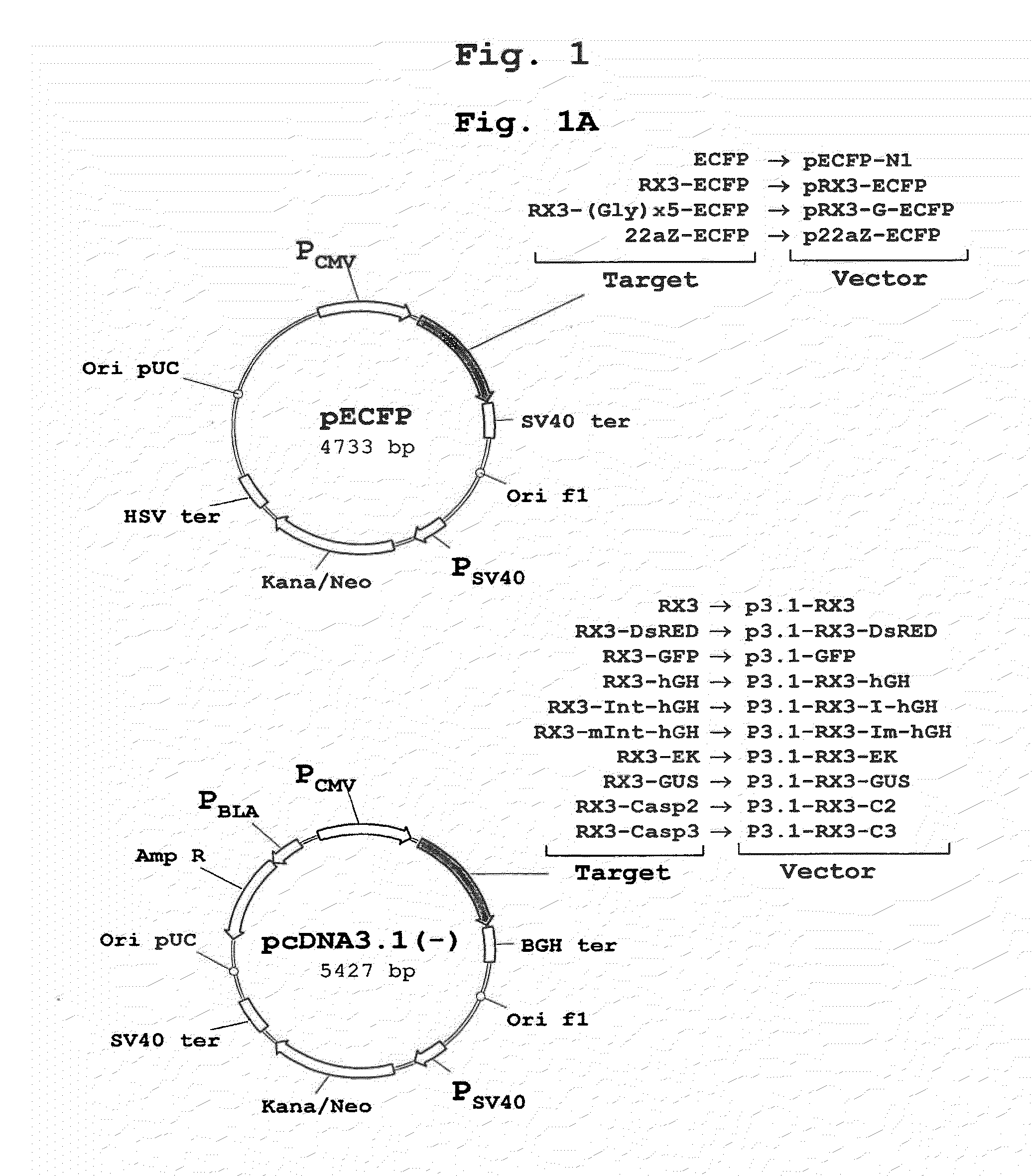
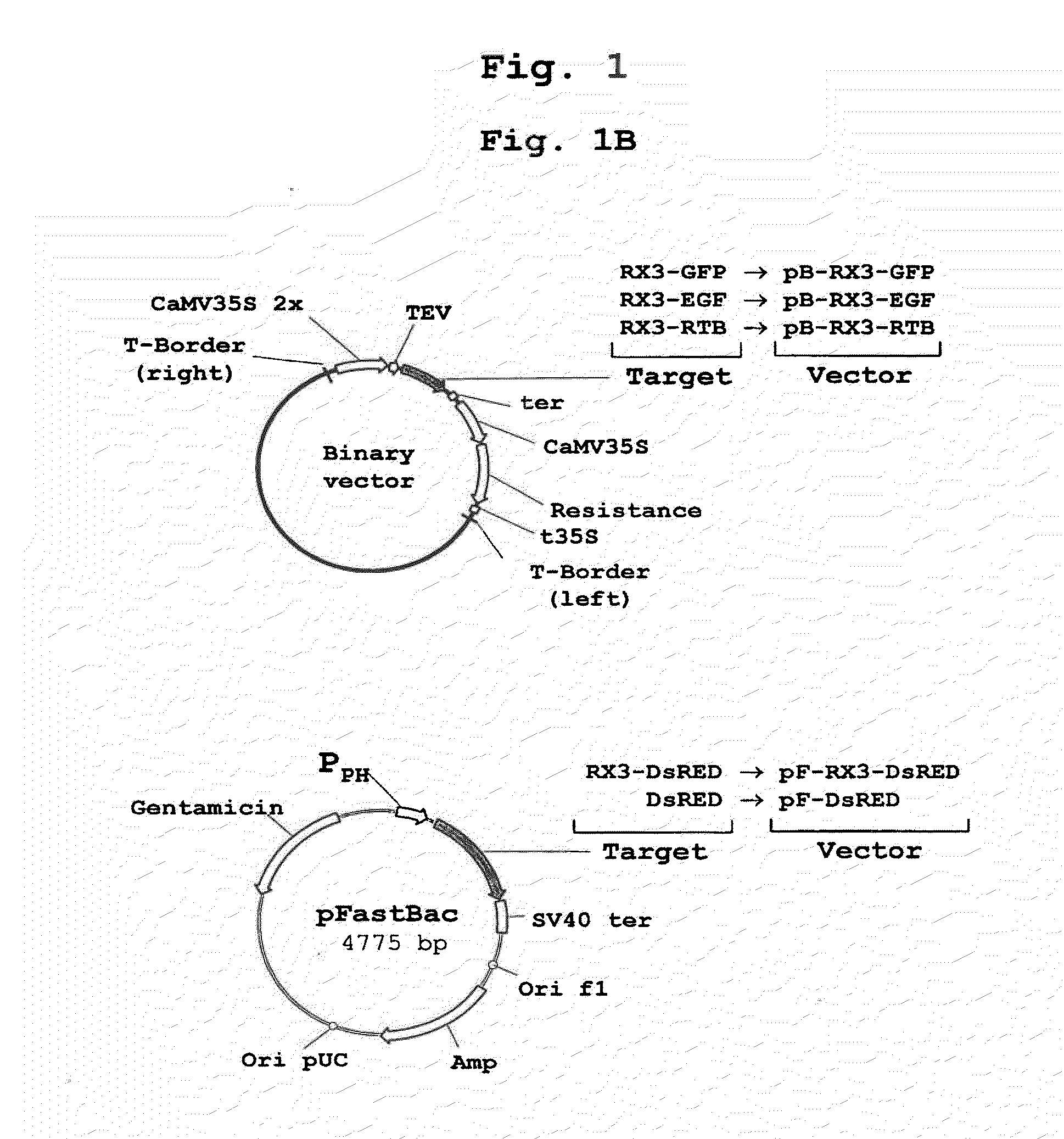
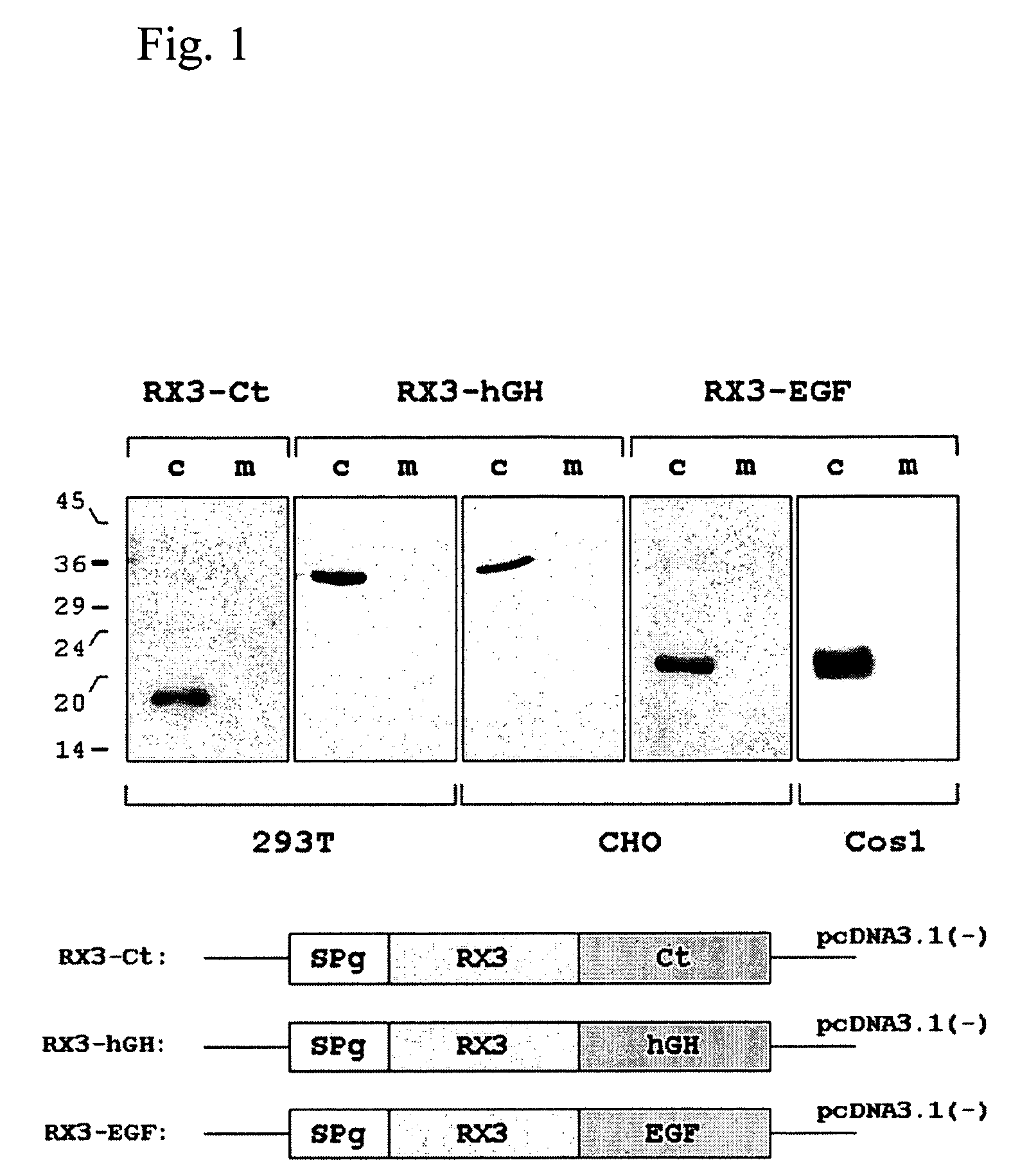
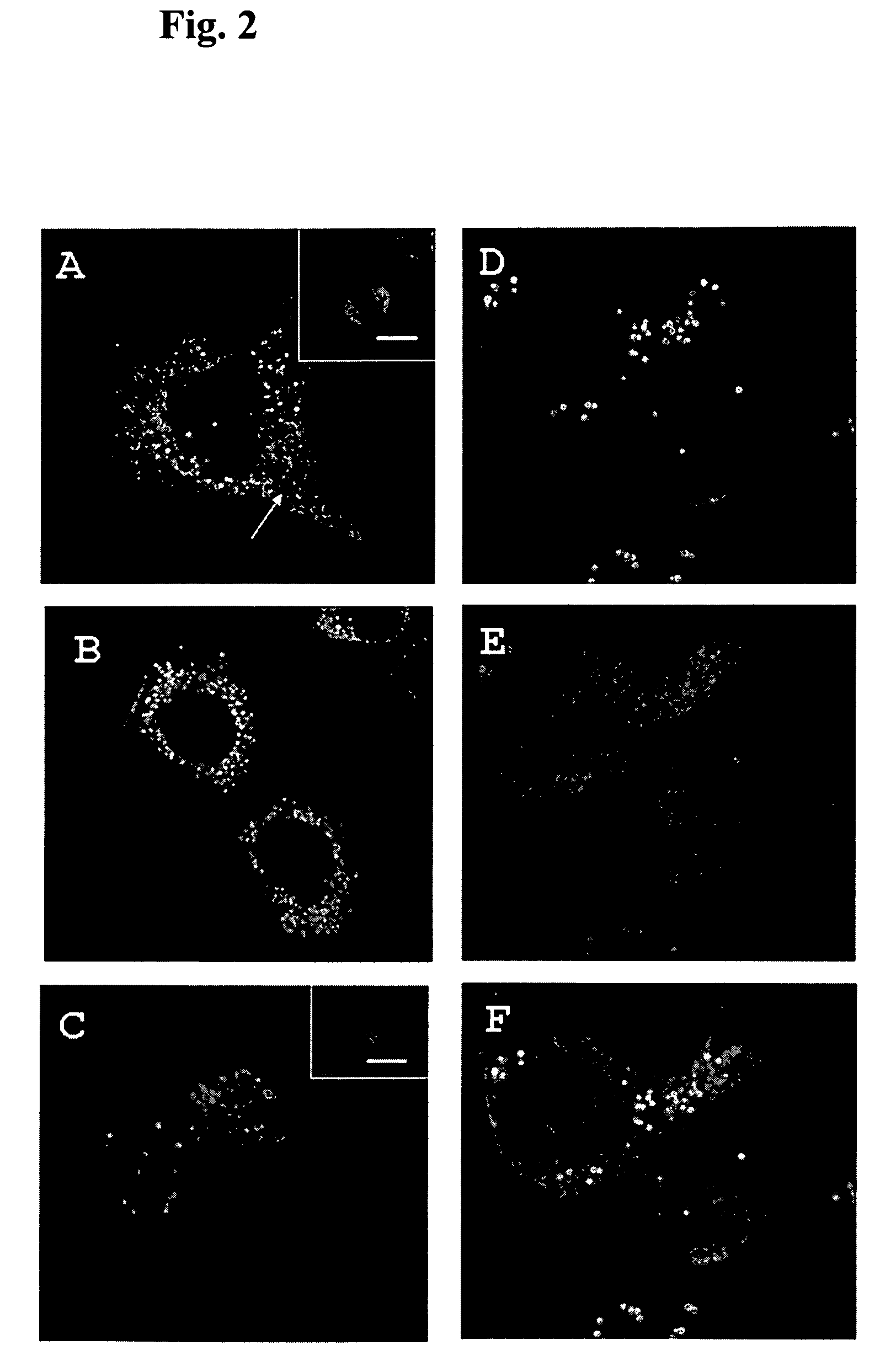
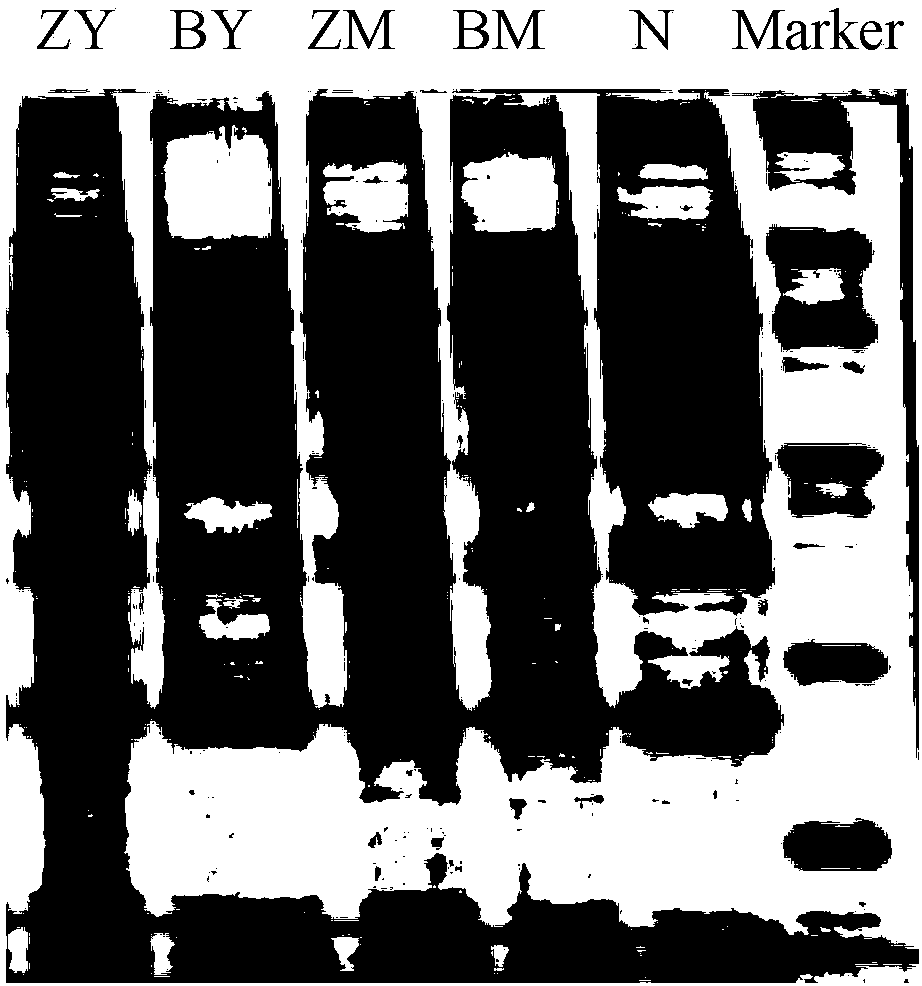
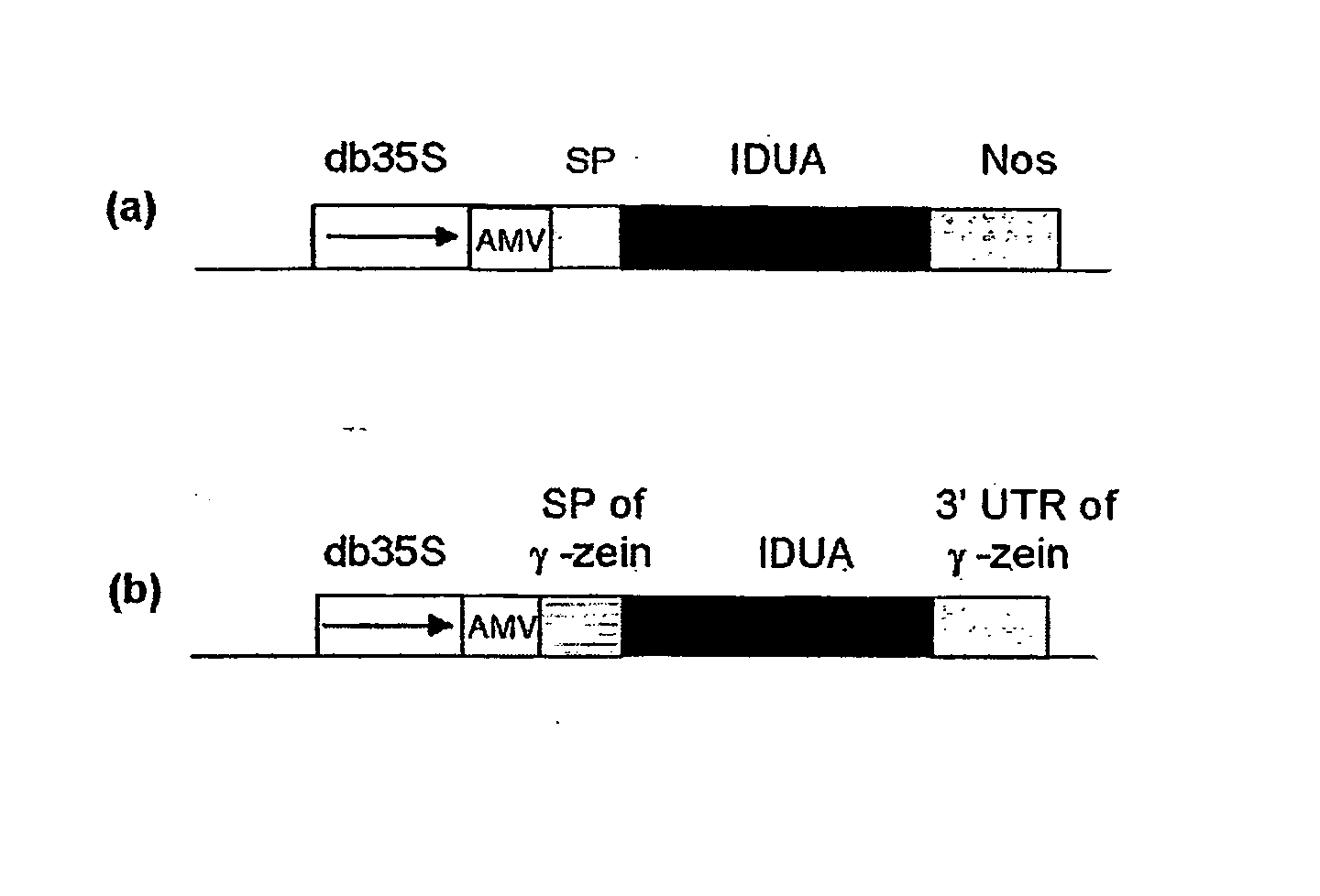
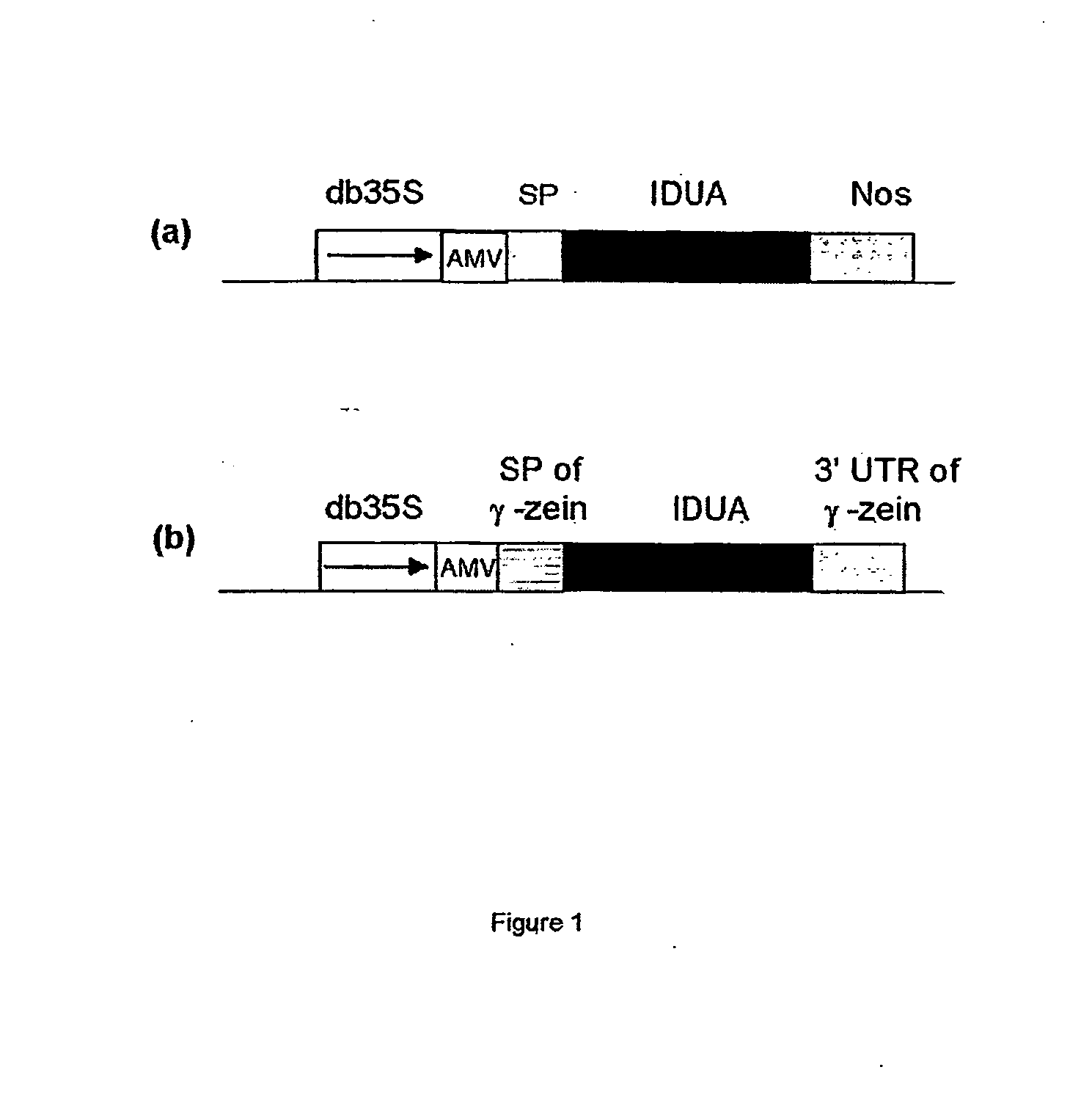
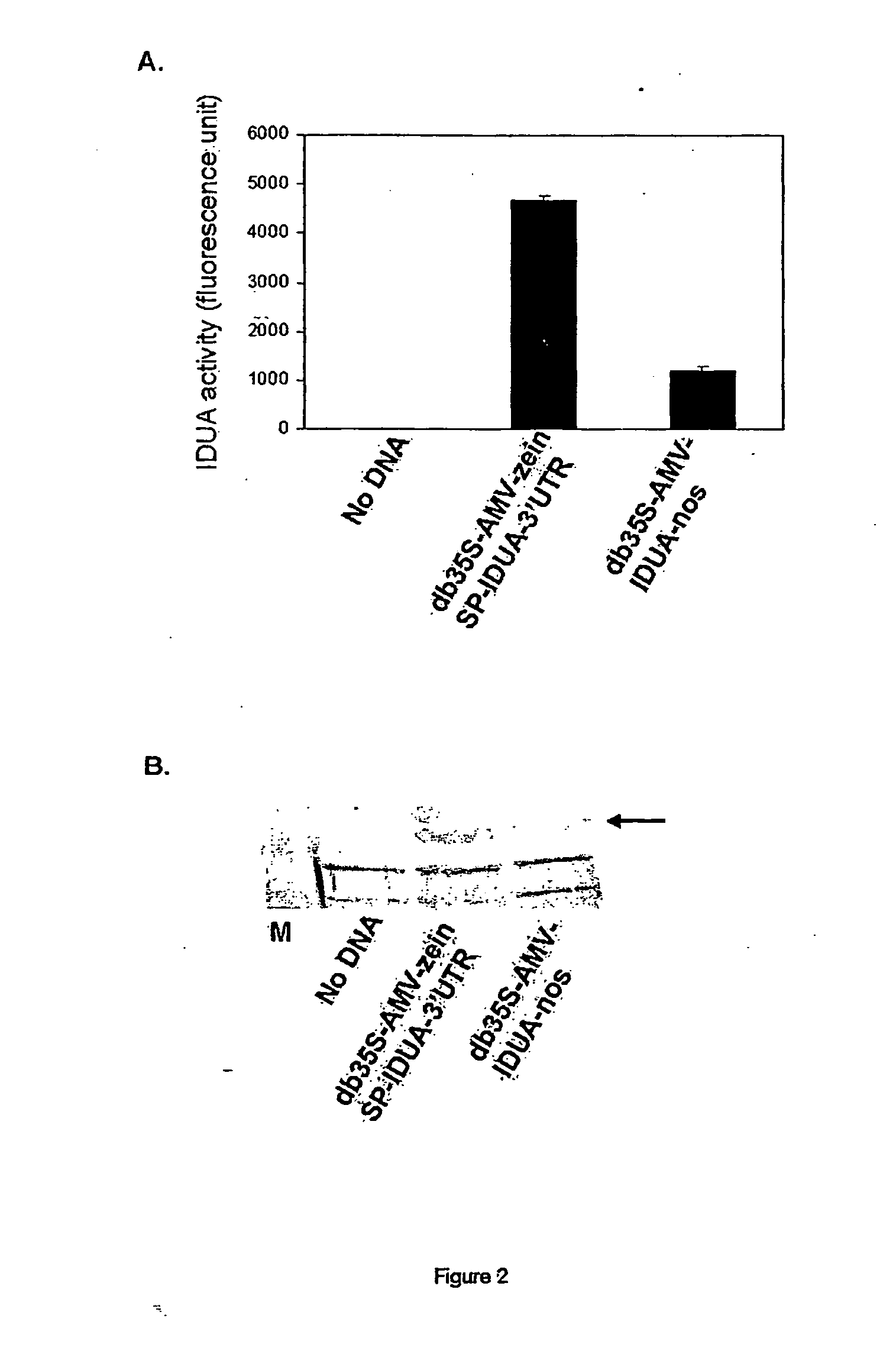
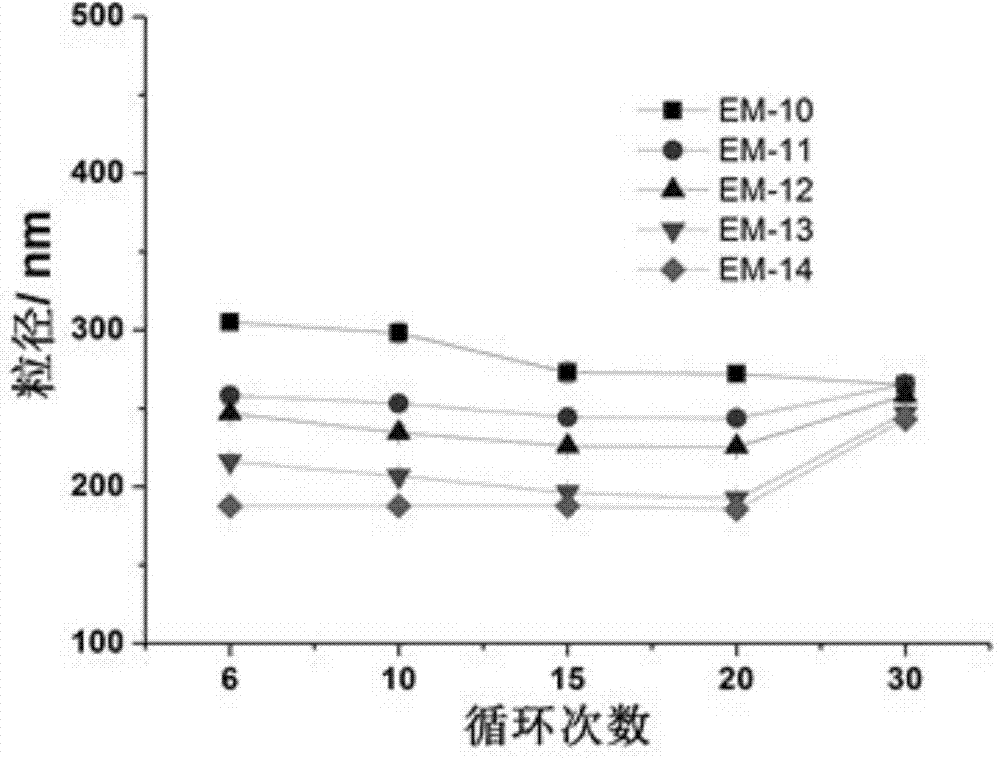
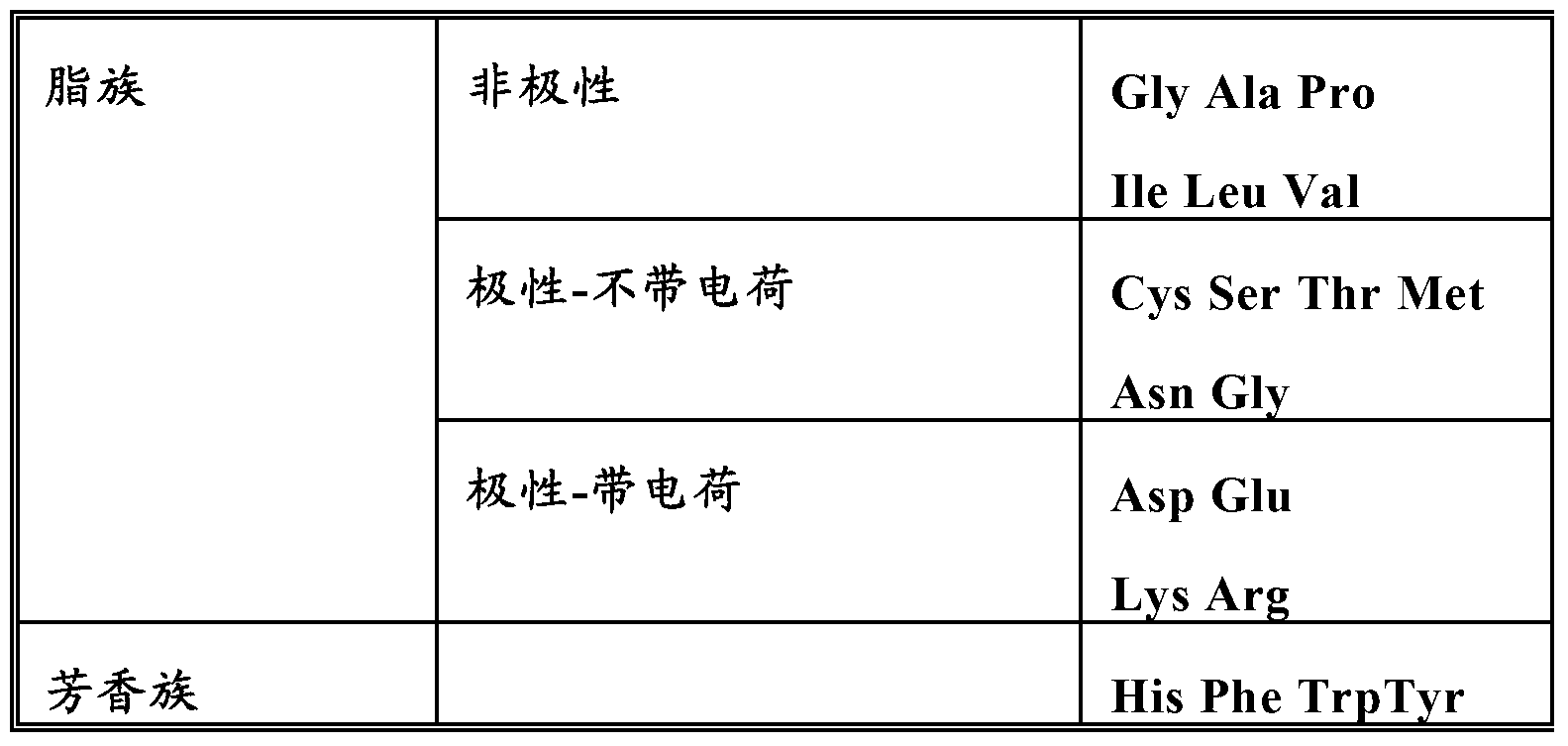
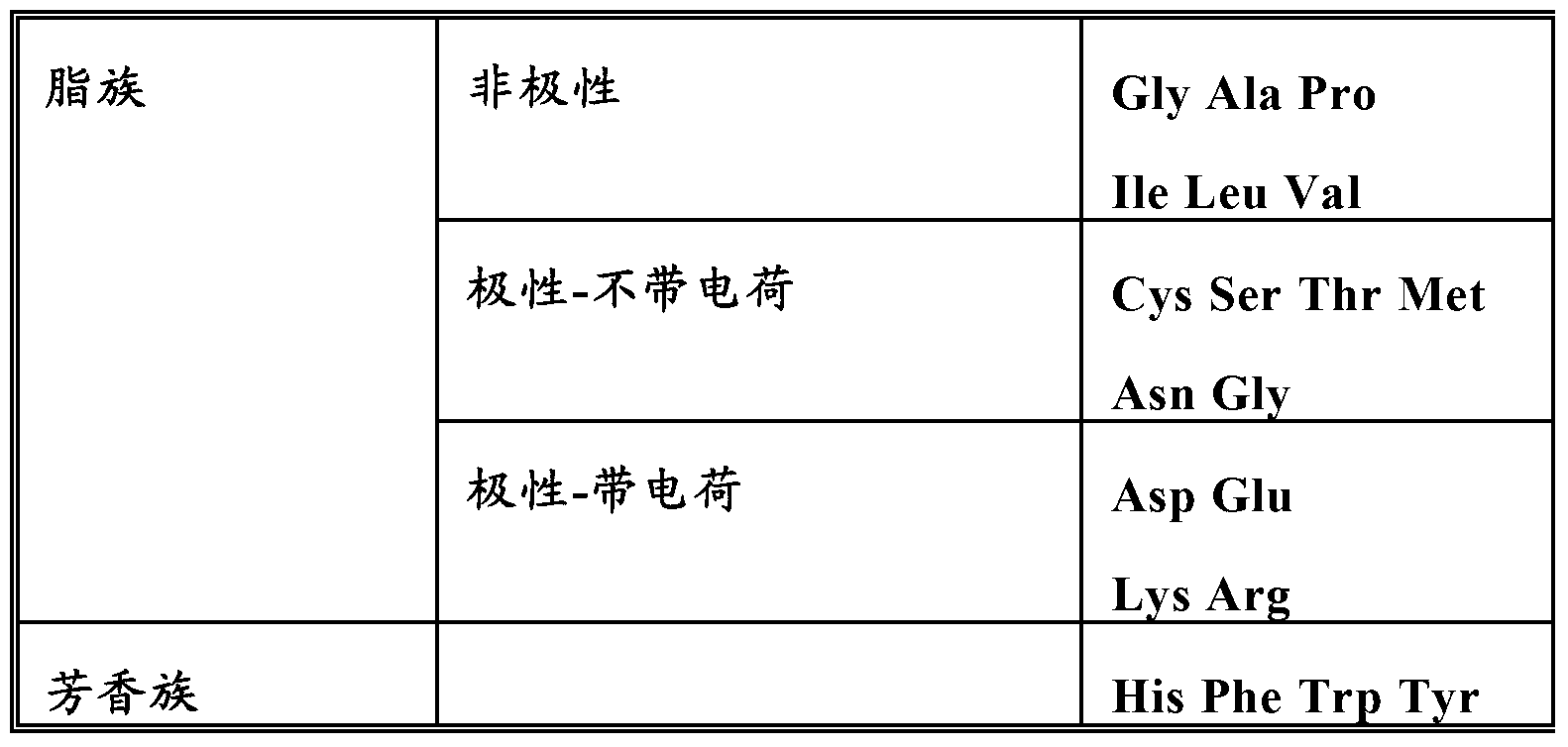
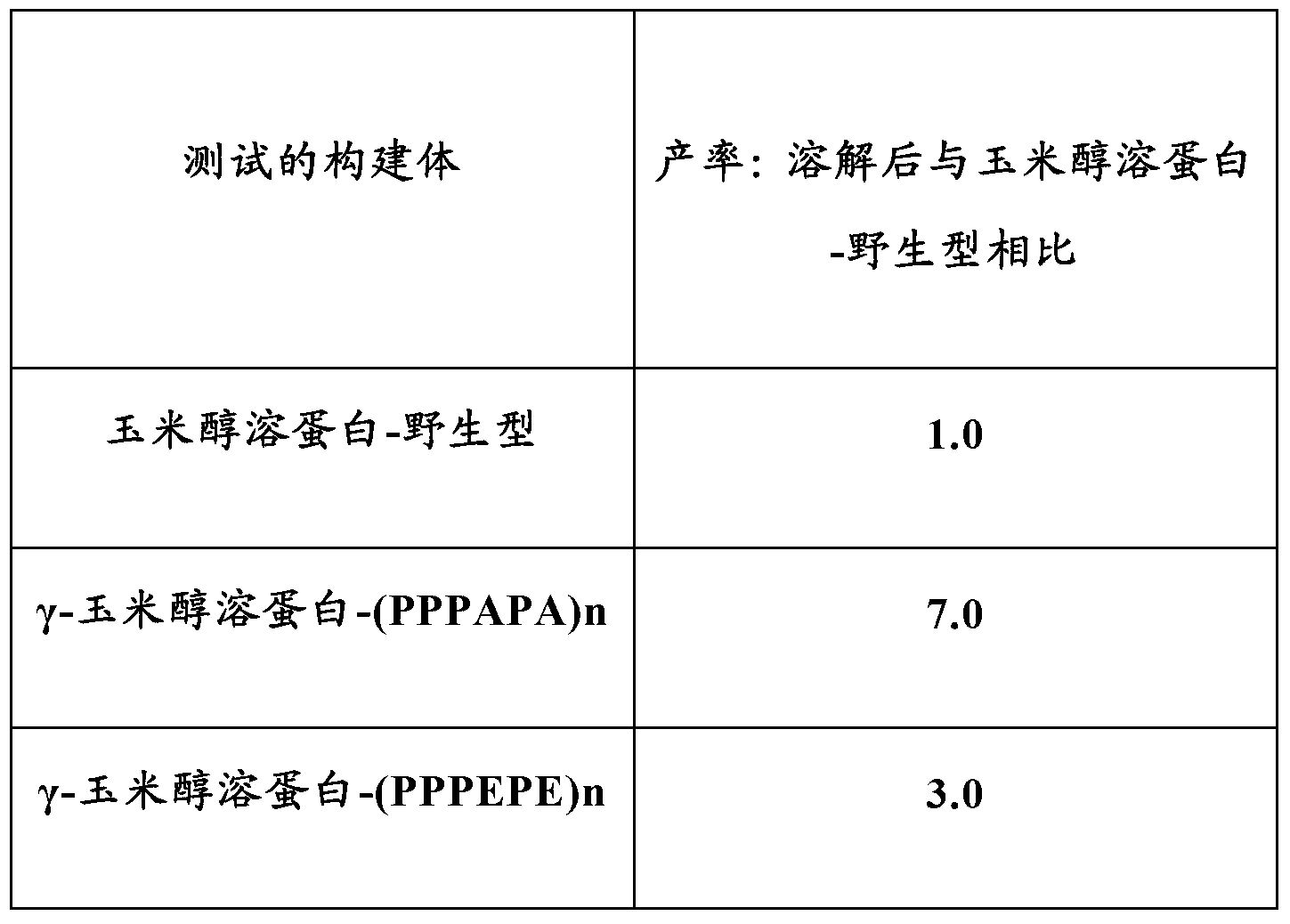
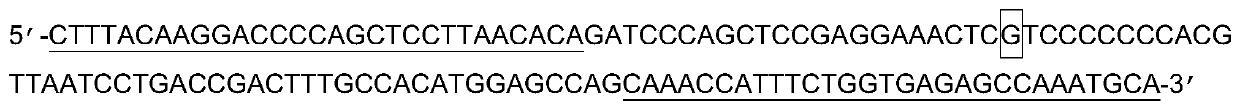Patents
Literature
98 results about "Protein body" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A membrane-bounded plant organelle found in the developing endosperm, contains storage proteins. [GOC:jl, PMID:7704047]
Zea mays transcription factor ZmbZIP22 and application thereof
ActiveCN107298701AReduce accumulationIncrease methionine contentPlant peptidesFermentationWild typeEssential amino acid
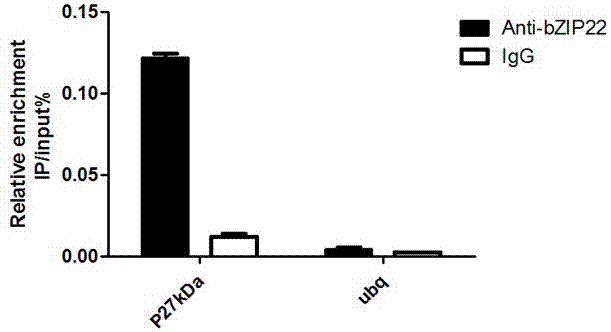
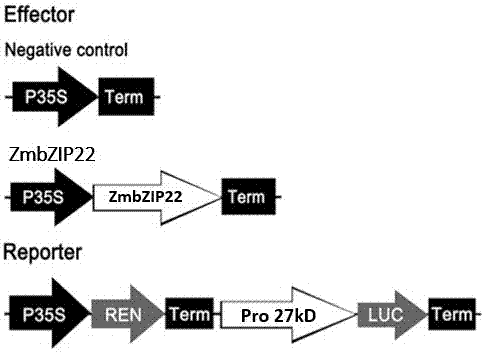
The invention relates to an application of a Zea mays grain transcription factor in the aspect of regulation and control of alcohal-soluble proteins. The factor comprises a base sequence shown in SEQ ID NO: 1. The protein ZmbZIP22 encoded by the sequence can be directly bonded to a 27kDa gamma-gliadin promoter and activate the 27kDa gamma-gliadin promoter. Gene-deficient mutant plants are obtained through carrying out Zea mays immature-embryo conversion by taking a gene fragment, shown in SEQ ID NO: 2, of ZmbZIP22 as a guide RNA by using a CRISPR-Cas9 technology. Compared with wild type grains, transgenic mutants have Zea mays grains with irregular and relative-thin protein body shells, the content of alcohal-soluble proteins in mature grains is remarkably lowered, the content of essential amino acids such as lysine, which are deficient to the conventional Zea mays, in the mature grains is remarkably increased, and thus, the nutritional quality of Zea mays is improved.
Owner:SHANGHAI UNIV
Recombinant Protein Body-Inducing Polypeptides
Polypeptide sequences for inducing recombinant protein bodies are described. The sequences comprise a polyproline II (PPII) structure and / or a proline-rich sequence between two cysteine residues on either end. Recombinant protein bodies are useful for protein production because they allow for simple and efficient purification of high quantities of recombinant protein. In addition, other methods of using recombinant protein bodies, for example, in vaccination and food products, are also described.
Owner:ERA BIOTECH SA
Production of proteins
A method for forming a fusion protein that is expressed as a recombinant protein body-like assembly in host eukaryotic cells and organisms other than higher plants as host systems is disclosed. More particularly, peptides and proteins are fused to protein sequences that mediate the induction of recombinant protein body-like assembly (RPBLA) formation, are stably expressed and accumulated in these host cells after transformation with an appropriate vector. Methods for preparing the fusion protein are also disclosed.
Owner:ERA BIOTECH SA
Production of biologically active proteins
ActiveUS20070243198A1Readily obtainableReadily purifiableVirusesHydrolasesBiological bodyActive protein
A fusion protein that is expressed in a recombinant protein body-like assembly (RPBLA) in host eukaryotic cells and organisms is disclosed. More particularly, a biologically active polypeptide fused to a protein sequence that mediates the induction of RPBLA formation is expressed and accumulated in host cells after transformation with an appropriate vector. The eukaryotic host cell does not produce protein bodies in the absence of the fusion protein. Methods for preparing and using the RPBLAs and the fusion protein are also disclosed, as are nucleic acid molecules that encode the fusion proteins.
Owner:ERA BIOTECH SA
Protein isolation and purification
InactiveUS20060123509A1Improve concentrationHigh densityFungiAntibody mimetics/scaffoldsVolumetric Mass DensityProtein C
A method for purifying a recombinant fusion protein that is expressed as recombinant protein body-like assemblies (RPBLAs) in host cells is disclosed in which an aqueous homogenate of transformed host cells that express a fusion protein as RPBLAs having a predetermined density is provided. Regions of different density are formed in the homogenate to provide a region that contains a relatively enhanced concentration of the RPBLAs and a region that contains a relatively depleted concentration of the RPBLAs. The RPBLAs-depleted region is separated from the region of relatively enhanced concentration of RPBLAs, thereby purifying said fusion protein. The region of relatively enhanced concentration of RPBLAs can thereafter be collected as desired.
Owner:ERA BIOTECH SA
Production of peptides and proteins by accumulation in plant endoplasmic reticulum-derived protein bodies
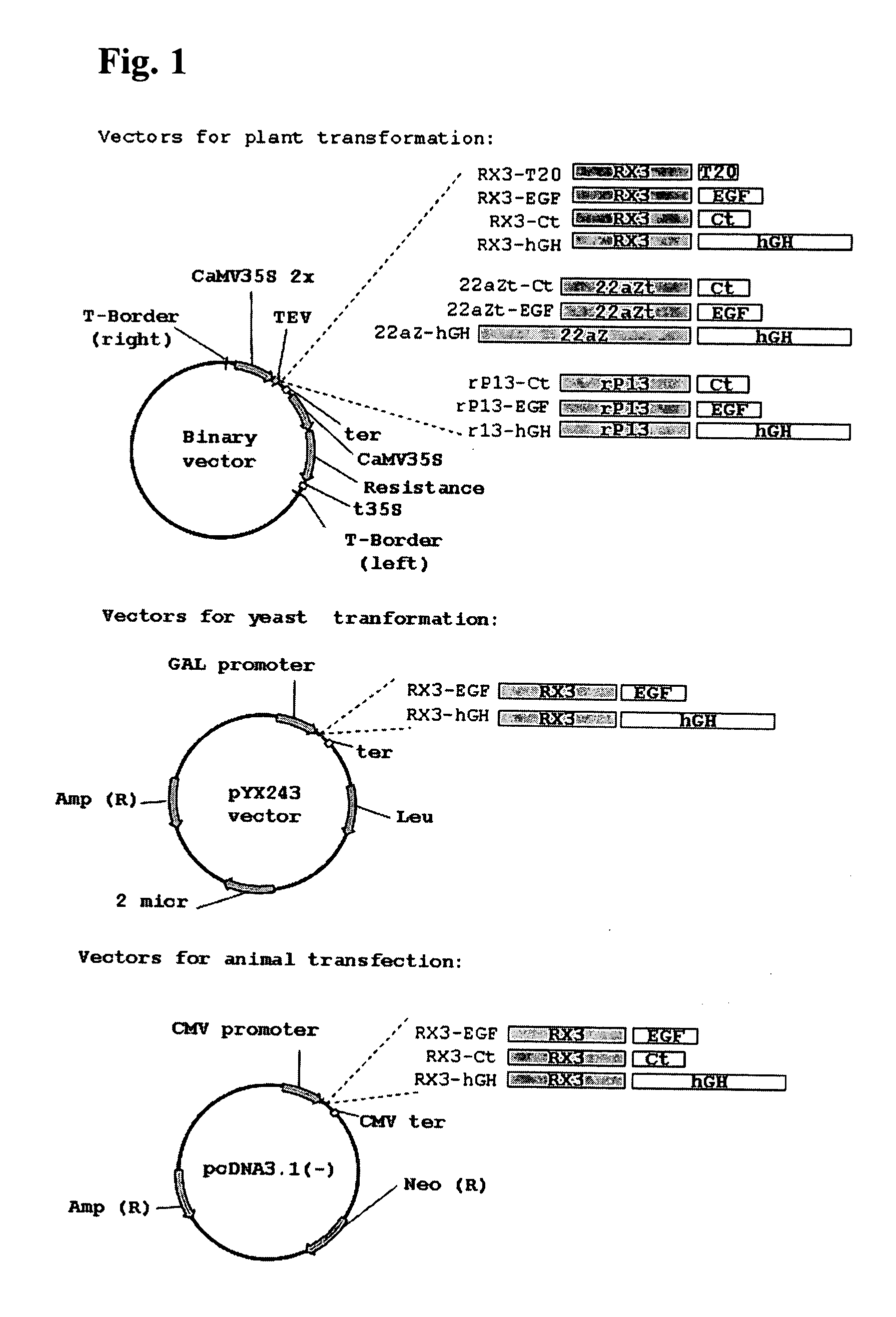
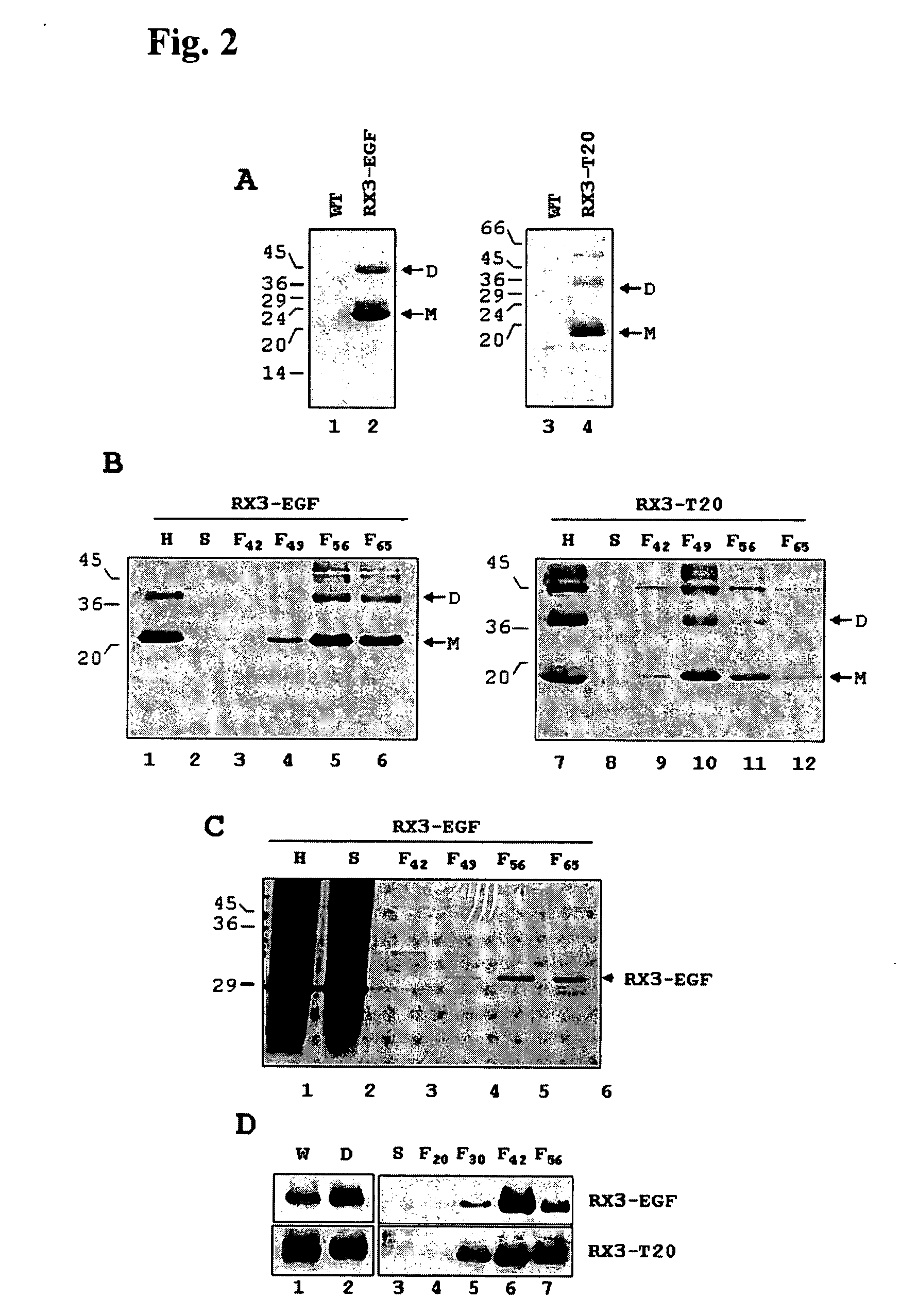
A nucleic acid molecule is disclosed as containing a first nucleic acid sequence comprising a nucleotide sequence that encodes γ-zein protein, or a fragment thereof capable of directing and retaining a protein towards the endoplasmic reticulum (ER) of a plant cell; a second nucleic acid sequence containing a nucleotide sequence that encodes an amino acid sequence that is specifically cleavable by enzymatic or chemical means; and a third nucleic acid sequence containing the nucleotide sequence that encodes a peptide or protein of interest. Methods of using this nucleic acid molecule for transforming host plant cells and producing the peptide or protein of interest are also disclosed.
Owner:ERA BIOTECH SA
Human occludin antigen epitope peptide, antigen, antibody, kit and use
ActiveCN108084257AGood antigenicityStrong specificityCell receptors/surface-antigens/surface-determinantsImmunoglobulins against cell receptors/antigens/surface-determinantsEpitopeOccludin
The present invention relates to a human occludin antigen epitope peptide, a human occludin antigen, a human occludin antibody, a kit and use. An amino acid sequence of the human occludin antigen epitope peptide is as shown in one of sequence table SEQ ID NO. 1 and sequence table SEQ ID NO.2. The human occludin antigen is prepared from the human occludin antigen epitope peptide and a protein carrier by coupling. A human occludin monoclonal or polyclonal antibody is prepared from the human occludin antigen. The human occludin monoclonal or polyclonal antibody is used for the preparation of an occludin in-vitro diagnostic kit. The human occludin antigen epitope peptide has good antigenicity, highly specific monoclonal and polyclonal antibodies can be produced by use of an antigen (immunogen)produced from the human occludin antigen epitope peptide for immunizing an animal, and the human occludin antigen epitope peptide can be applied to human occludin in vitro testing.
Owner:深圳市安群生物工程有限公司
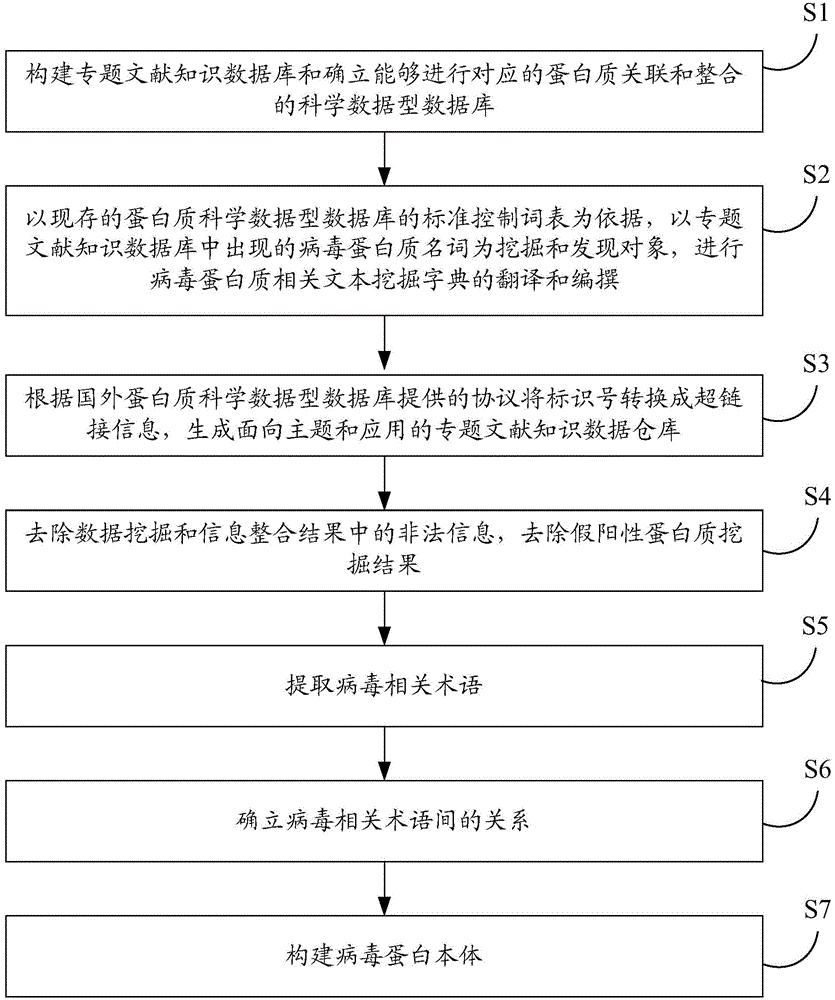
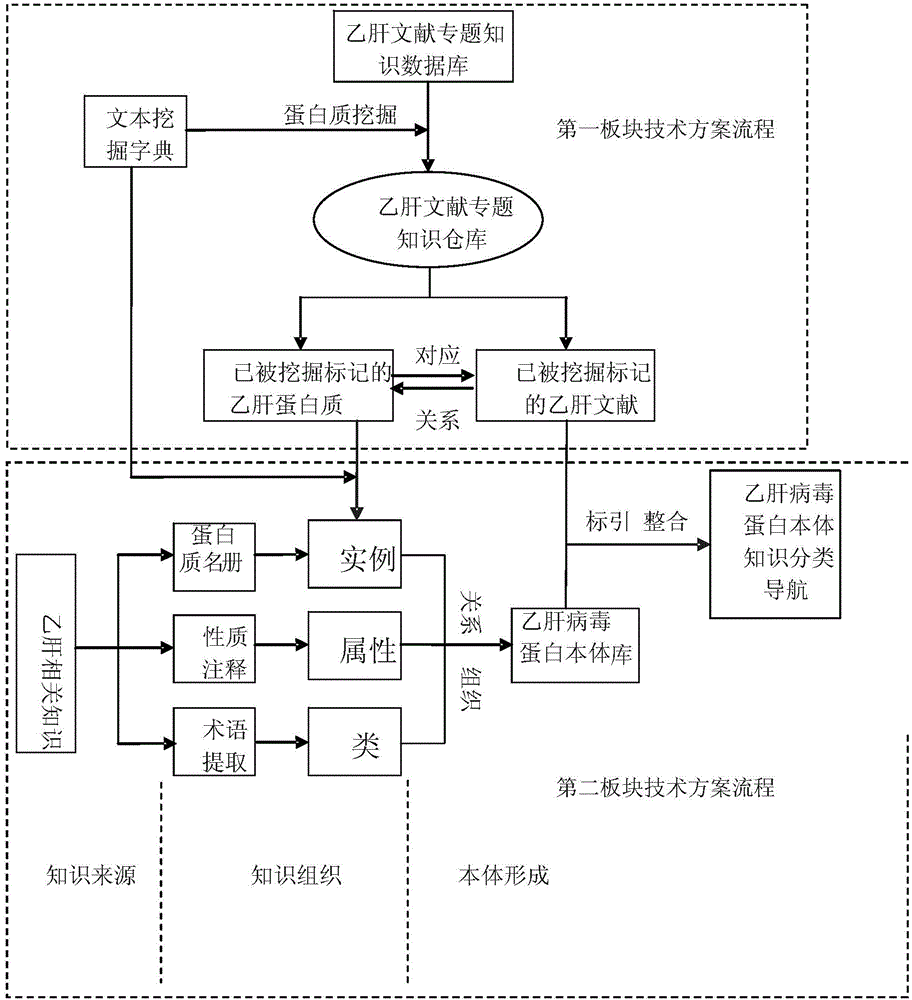
Knowledge navigation method, device and system based on virus protein body
ActiveCN104424399AQuickly and accurately obtainSearch results are accurateSpecial data processing applicationsText miningVirus Protein
The invention discloses a knowledge navigation method, device and system based on a virus protein body. On the basis of achieving the knowledge excavation, the knowledge navigation method based on the virus protein body is created, and the integration of the knowledge excavation and the knowledge navigation function is well completed. According to the technical scheme, the relevance integration is conducted on a text mining dictionary of a knowledge excavation type hepatitis B special subject literature database and the hepatitis B virus protein body, a knowledge organization and classification navigation system applied to a hepatitis B protein excavation module is established through the relevance integration, and the knowledge navigation function based on the hepatitis B virus protein body is completed.
Owner:SHANGHAI INST OF BIOLOGICAL SCI CHINESE ACAD OF SCI
Oral or intranasal vaccines using hydrophobic complexes having proteosomes and lipopolysaccharides
InactiveUS20020037295A1Promote intestinal absorptionAvoid contactAntibacterial agentsBacterial antigen ingredientsSerum igeIntestinal fluid
An immunogenic complex, essentially consisting of neisserial outer membrane protein proteosomes hydrophobically complexed to native purified bacterial lipopolysaccharide and formulated in accordance with the current invention for mucosal delivery such as via the oral or intranasal route is used as a vaccine. Specifically, a vaccine using shigella lipopolysaccharides complexed to proteosomes for such mucosal administration induces IgG and IgA antibodies in sera and in respiratory and intestinal fluids. Furthermore, such antibodies are associated with protection against shigella infection and these vaccines are herein demonstrated to protect against mucosal infection with shigella.
Owner:US ARMY MEDICAL RES MATERIEL COMMAND USAMRMC
Research method for classifying endosperm tissues in detail by using morphological method
InactiveCN104390978AAbundant evidenceComplete informationScanning probe techniquesFluorescence/phosphorescenceSoftware development processFluorescence
The invention discloses a research method for classifying endosperm tissues in detail by using a morphological method. The research method comprises the following steps: planting corn, sorghum, wheat and rice, experimenting caryopsis developed for different days; examining size, shape and color changes of the caryopsis by using an integrated microscope, and observing position and distribution changes of the endosperm tissues; observing fluorescence characteristics of different tissues of the caryopsis by using a luminescence microscope, and analyzing ingredients of the caryopsis; observing microstructure change of caryopsis and endosperm tissue development by using a light microscope; observing a three-dimensional shape and distribution of cell walls, amyloid, protein body and aleurone cereal by using a scanning electron microscope; and observing characteristics and changes of subcellular structures in cells by using a transmission electron microscope. According to the research method, by using four main cereals as experiment materials, a total development process of the cereal endosperm is analyzed and verified by using various morphological observation technologies, an important role of the endosperm during development of the caryopsis is displayed, and important information is provided for evolution development by comparing structural difference of different grain endosperms.
Owner:YANGZHOU UNIV
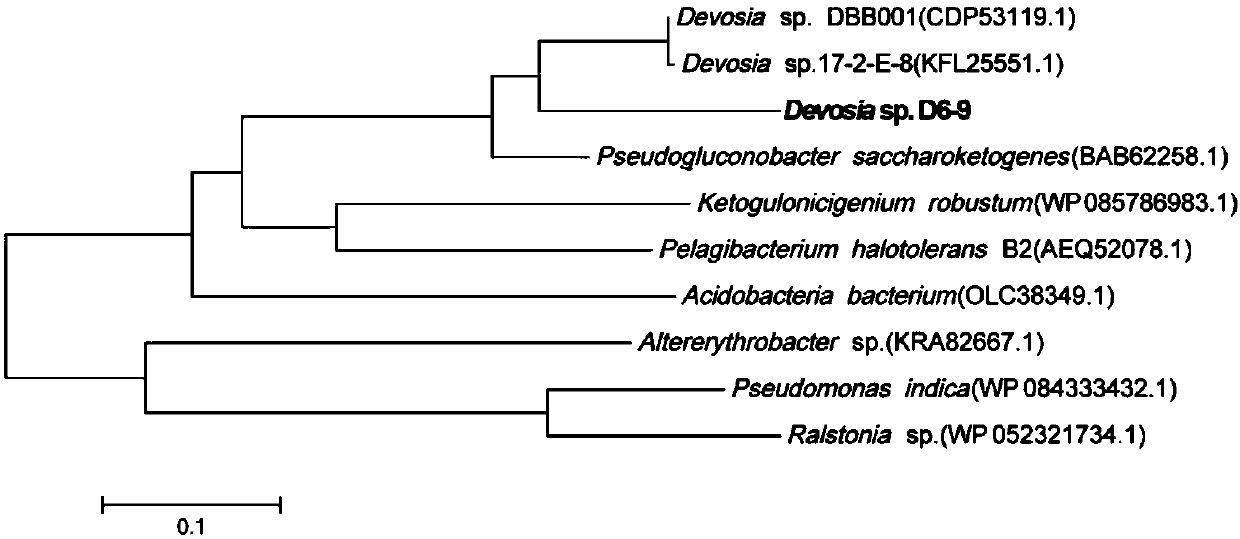
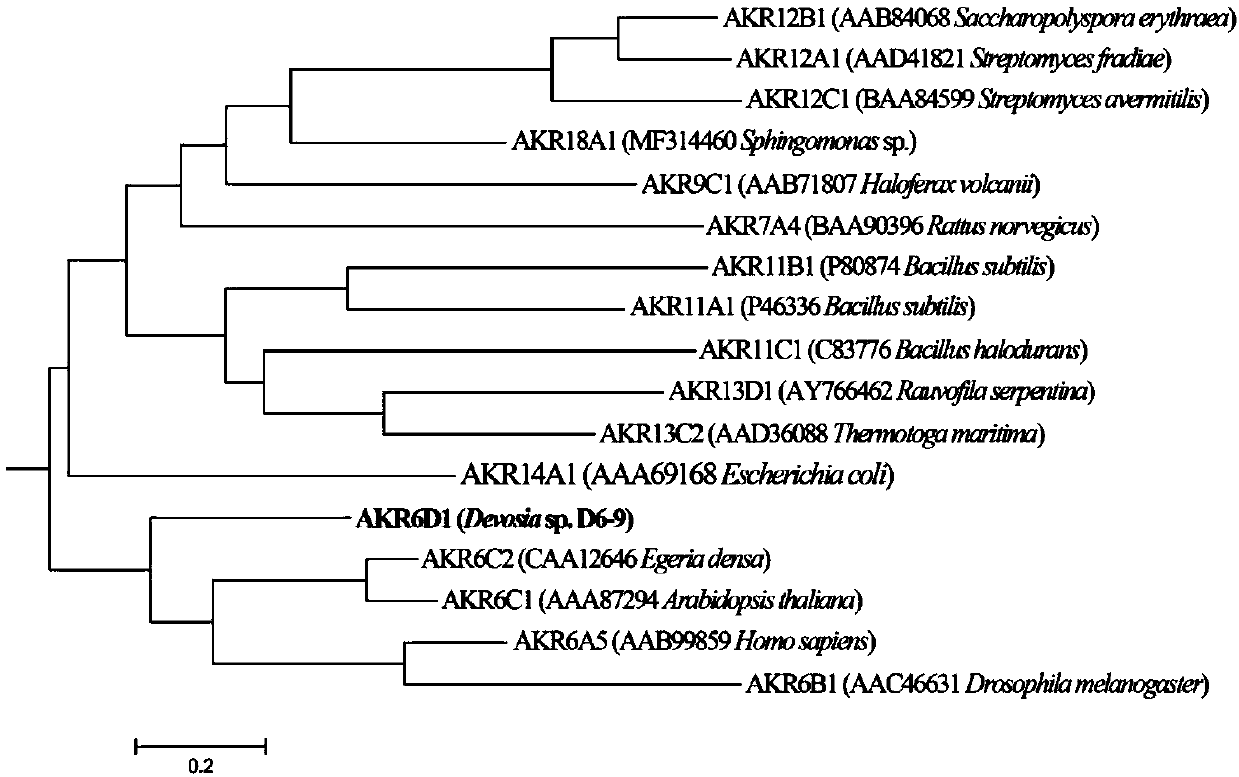
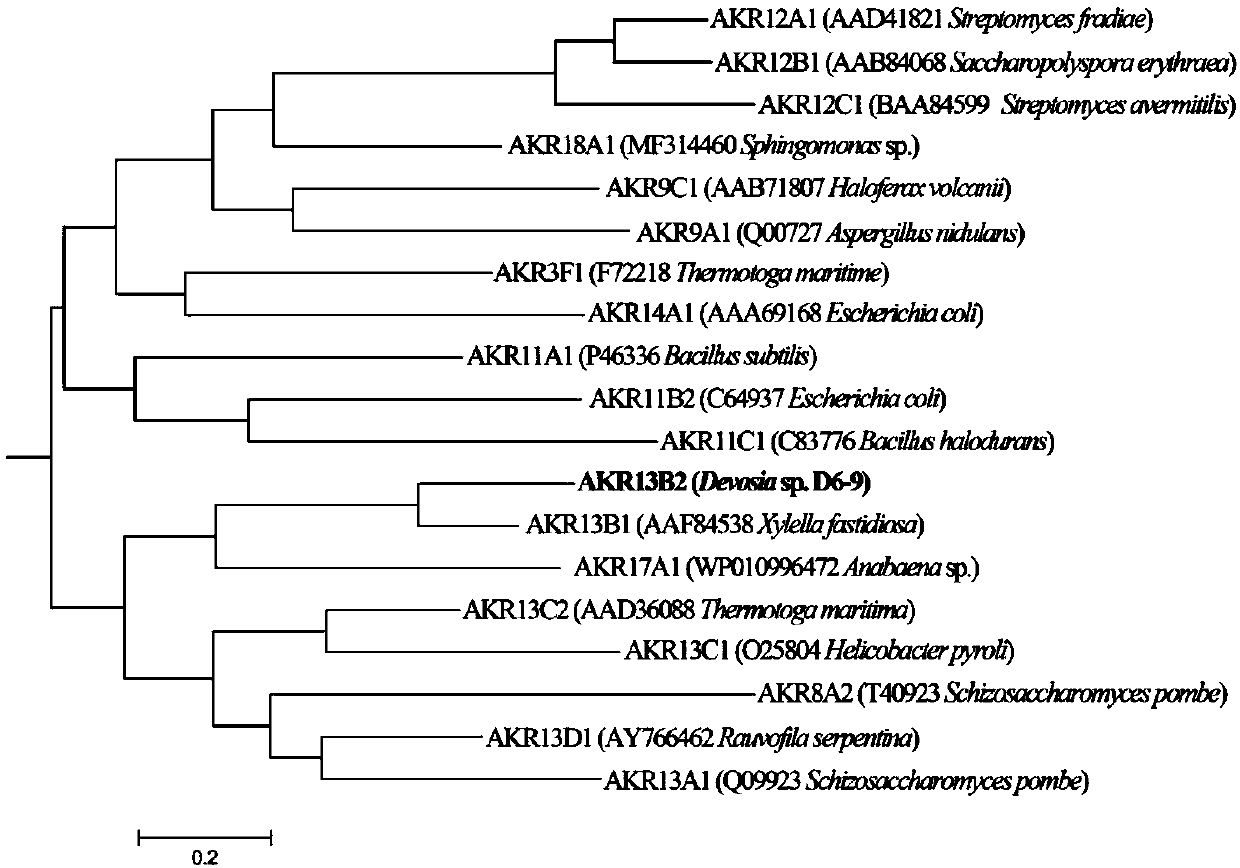
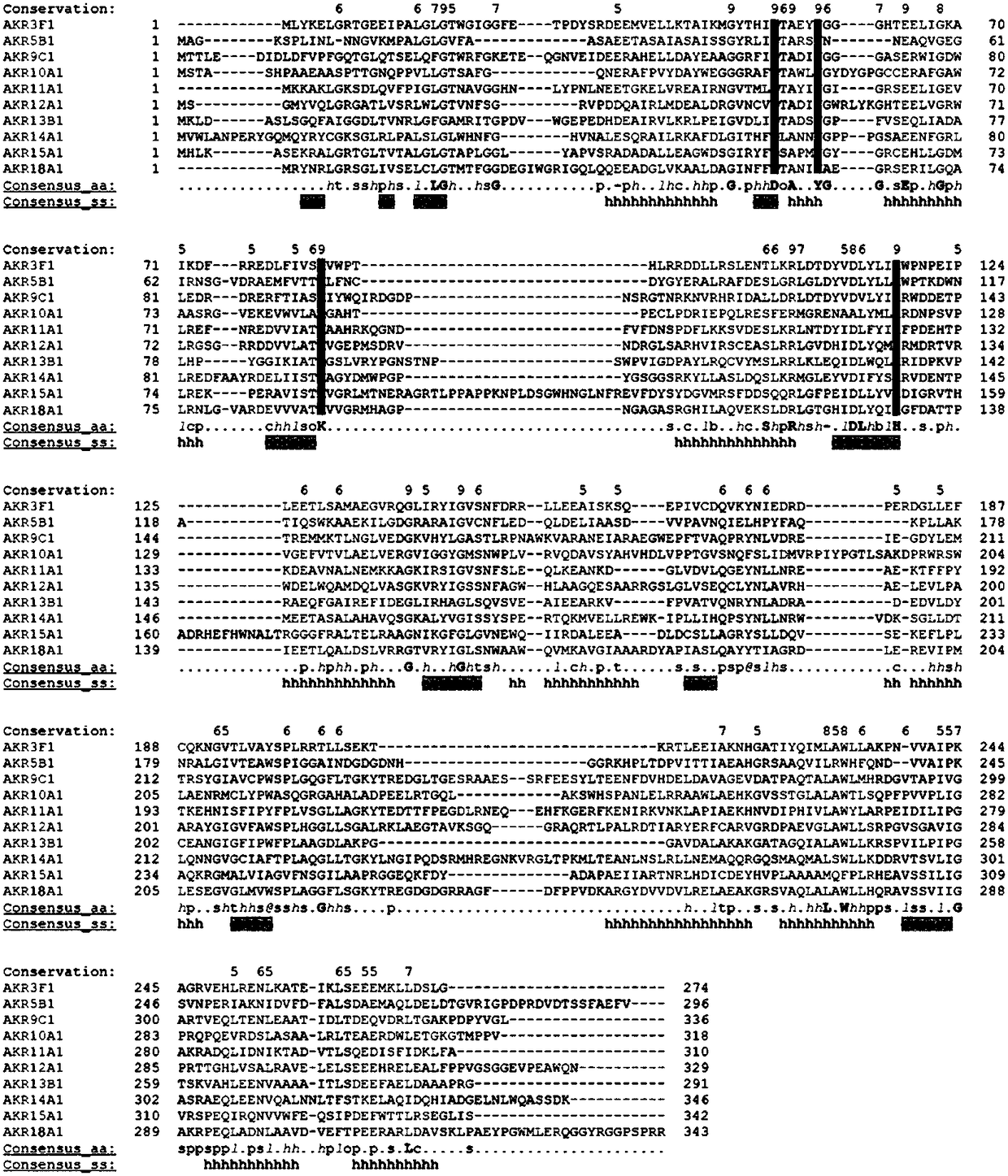
Fusarium toxin removal path related genes ADH, AKR6D1 and AKR13B2 and their application
ActiveCN107916266AHigh expressionImprove solubilityOxidoreductasesFermentationProkaryotic expressionToxin
The invention discloses Fusarium toxin removal path related genes ADH, AKR6D1 and AKR13B2 and their application. Purified proteins are acquired by prokaryotic expression of the genes ADH, AKR6D1 and AKR13B2; in-vitro experiments prove that the three purified proteins can jointly catalyze DON (deoxynivalenol) to form 3-isomer-deoxynivalenol (3-eip-DON), wherein the protein ADH can oxidize DON to form 3-oxo-deoxynivalenol (3-oxo-DON), and the proteins AKR6D1 and AKR13B2 can further reduce 3-oxo-DON to form 3-epi-DON having lowest toxicity presently known.
Owner:HUAZHONG AGRI UNIV
Production of Biologically Active Proteins
InactiveUS20120020992A1Readily obtainableReadily purifiableVirusesPharmaceutical delivery mechanismBiological bodyActive protein
A fusion protein that is expressed in a recombinant protein body-like assembly (RPBLA) in host eukaryotic cells and organisms is disclosed. More particularly, a biologically active polypeptide fused to a protein sequence that mediates the induction of RPBLA formation is expressed and accumulated in host cells after transformation with an appropriate vector. The eukaryotic host cell does not produce protein bodies in the absence of the fusion protein. Methods for preparing and using the RPBLAs and the fusion protein are also disclosed, as are nucleic acid molecules that encode the fusion proteins.
Owner:ERA BIOTECH SA
Production of proteins
InactiveUS8822181B2Readily obtainable and purifiableCalcitoninsUnicellular algaeEisosome assemblyProtein
A method for forming a fusion protein that is expressed as a recombinant protein body-like assembly in host eukaryotic cells and organisms other than higher plants as host systems is disclosed. More particularly, peptides and proteins are fused to protein sequences that mediate the induction of recombinant protein body-like assembly (RPBLA) formation, are stably expressed and accumulated in these host cells after transformation with an appropriate vector. Methods for preparing the fusion protein are also disclosed.
Owner:ERA BIOTECH SA
Recombinant Protein Bodies as Immunogen-Specific Adjuvants
An immunogen-specific is adjuvant for a vaccine or inoculum is disclosed. The adjuvant is comprised of particulate recombinant protein body-like assemblies (RPBLAs) that contain a recombinant fusion protein that contains two portions peptide-linked together. A first portion is a protein body-inducing sequence (PBIS) and a second portion is a T-cell stimulating immunogenic polypeptide whose sequence is that of a pathogenic polypeptide sequence present in or induced by a vaccine or inoculum. The adjuvant, when used as an inoculum in a host animal without a prior priming vaccination or inoculation, does not induce production of antibodies or T cell activation to the pathogenic sequence.
Owner:ERA BIOTECH SA
Preparation of chicken eimeria tenella strain with EYFP knocked into Et.Actin gene
PendingCN113583876ANo distractionSolve pollutionProtozoaStable introduction of DNAAnticoccidial AgentsCecum mucosa
The invention discloses preparation of a chicken eimeria tenella strain with EYFP knocked into an Et.Actin gene. According to the invention, an FnCas12a protein, crRNA synthesized in vitro and a homologous recombination fragment (an Et.Actin gene containing a P2A-DHFR-EYFP expression cassette) are introduced into chicken eimeria tenella sporozoite by utilizing a Lonza 4D-Nucleofector nuclear transformation instrument, so that the editing of the chicken eimeria tenella is realized. The edited insect strain is screened through pyrimethamine in a chicken body to obtain a high-purity gene edited insect strain. Then, the gene edited insect strain is verified by utilizing PCR and Sanger sequencing technologies, and it is proved that the EYFP successfully marks the Et.Actin gene. After sporozoites of the strain are subjected to cell culture and a chicken is infected, cecum mucosa is taken and smeared to show that the EYFP is expressed in the whole life history of the gene edited strain. The gene editing method can be used for performing fixed-point marking on a chicken coccidiosis gene, and is beneficial to analyzing the function of the coccidiosis gene. The invention also has important significance for research and development of novel anticoccidial drugs and vaccines.
Owner:SHANGHAI VETERINARY RES INST CHINESE ACAD OF AGRI SCI
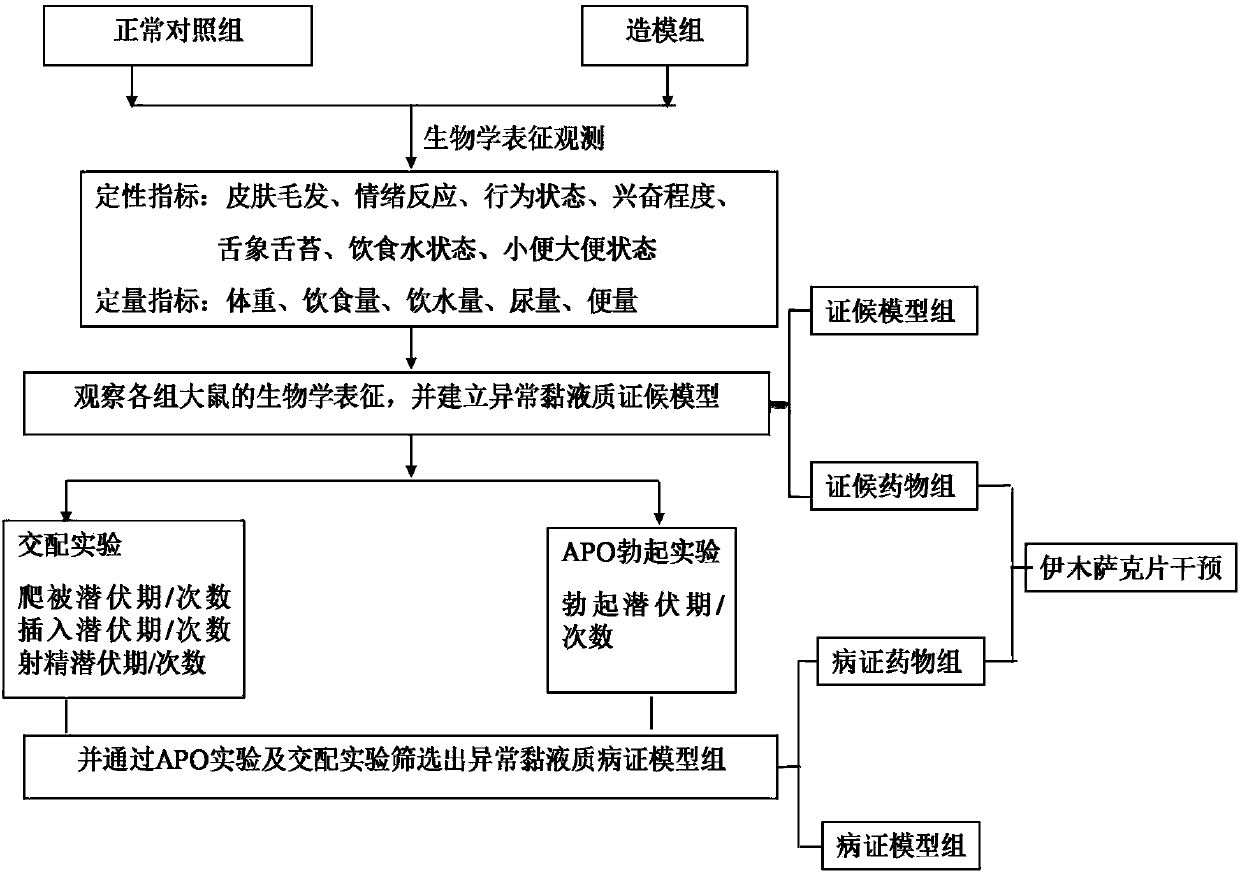
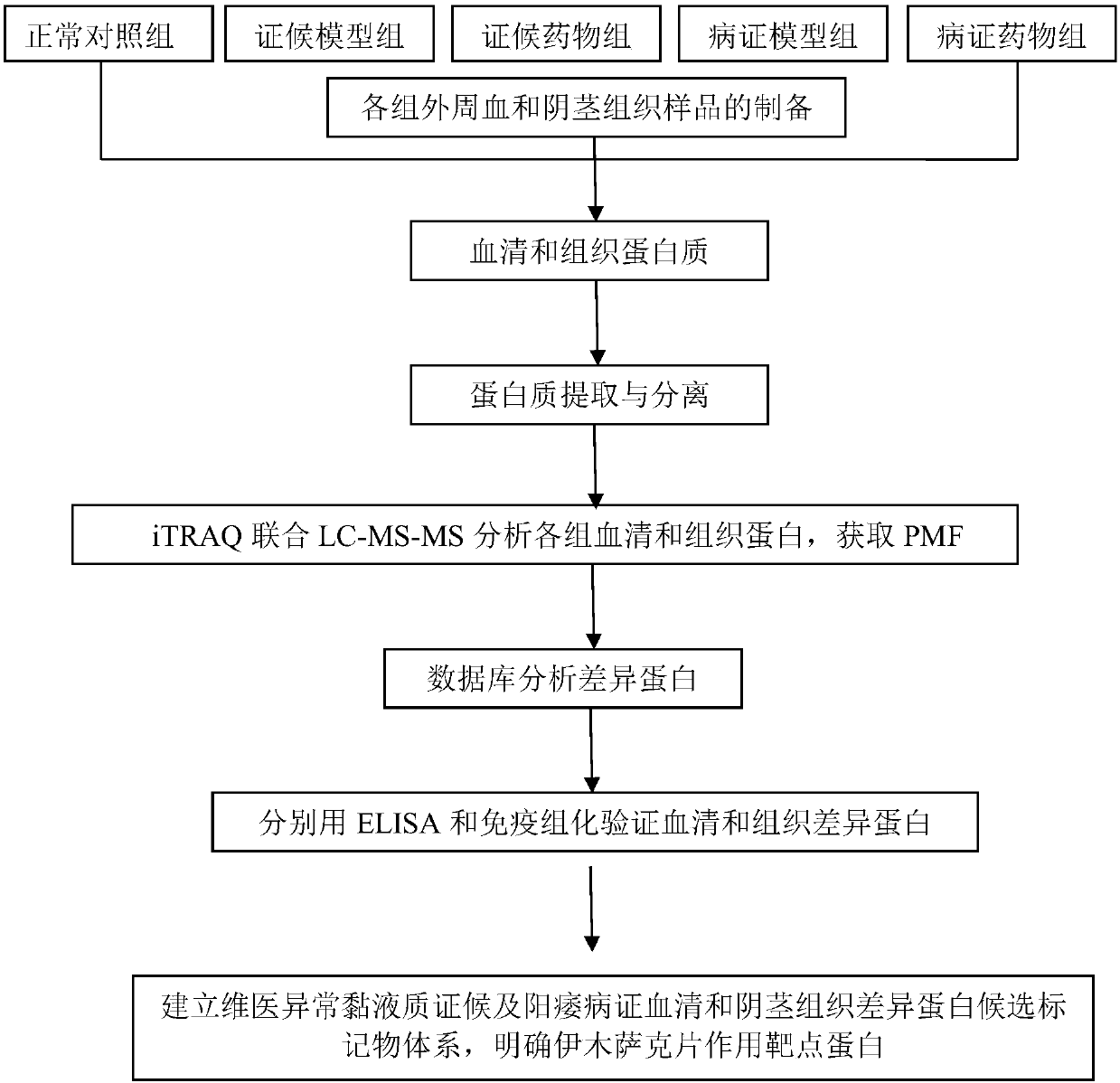
Target protein of Yimusake tablet acting on abnormal balham syndrome and erectile dysfunction syndrome models and screening method thereof
The invention provides medicine target protein of serums after a Yimusake tablet acts on rats with an abnormal balham syndrome and an abnormal Balham type erectile dysfunction disease, and a penis smooth muscle tissue, and a screening method thereof. The method comprises the following steps of 1, providing a normal comparison set, a syndrome set, a syndrome medicine set, a disease set and a disease medicine set; 2, collecting whole blood, separating the serum and the penis smooth muscle tissue from the whole blood; 3, extracting the protein; 4, purifying after marking; 5, acquiring a fingerprint spectrum after spectrum detection, analyzing protein compositions after screening, and acquiring each set of serums and the penis smooth muscle tissue protein; and 6, acquiring a Yimusake tablet action target protein system by making statistics, comparing and screening. According to the protein and the screening method of the protein provided by the invention, the action target of the uigur medicine, the Yimusake tablet, on the abnormal Balham type erectile dysfunction in uigur medicine is clear, and a foundation is laid for further disclosure of the action mechanism of the Yimusake tablet and new and secondary development of the Yimusake tablet.
Owner:XINJIANG MEDICAL UNIV
Production Of A Protein Localized To The Endoplasmic Reticulum Protein Bodies In A Transgenic Plant And Seed
InactiveUS20080034455A1Avoid it happening againFacilitates prolamin localizationSugar derivativesFermentationHeterologousReticulum cell
A nucleotide sequence is provided for expression of a heterologous protein of interest that is localized to the endoplasmic reticulum protein bodies in transgenic plants, plant cells, and seeds. Said nucleotide sequence comprises a promoter, the 5′ untranslated region from the maize γ-zein gene, the γ-zein signal peptide sequence, a sequence encoding a heterologous protein of interest, and the 3′ untranslated region from the maize γ-zein gene, operatively linked.
Owner:SIMON FRASER UNIVERSITY
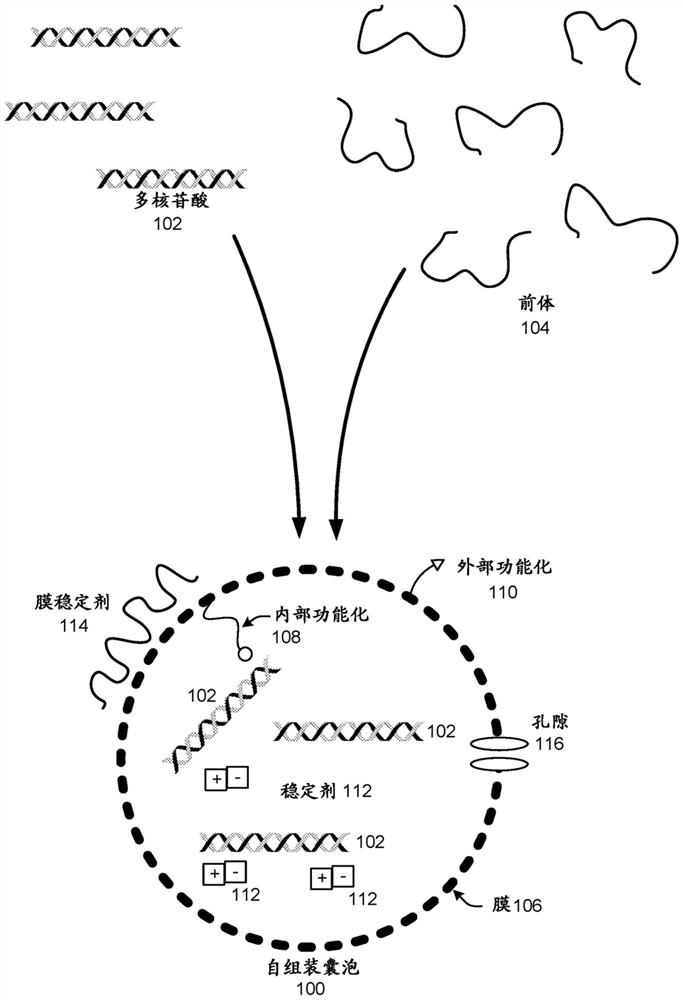
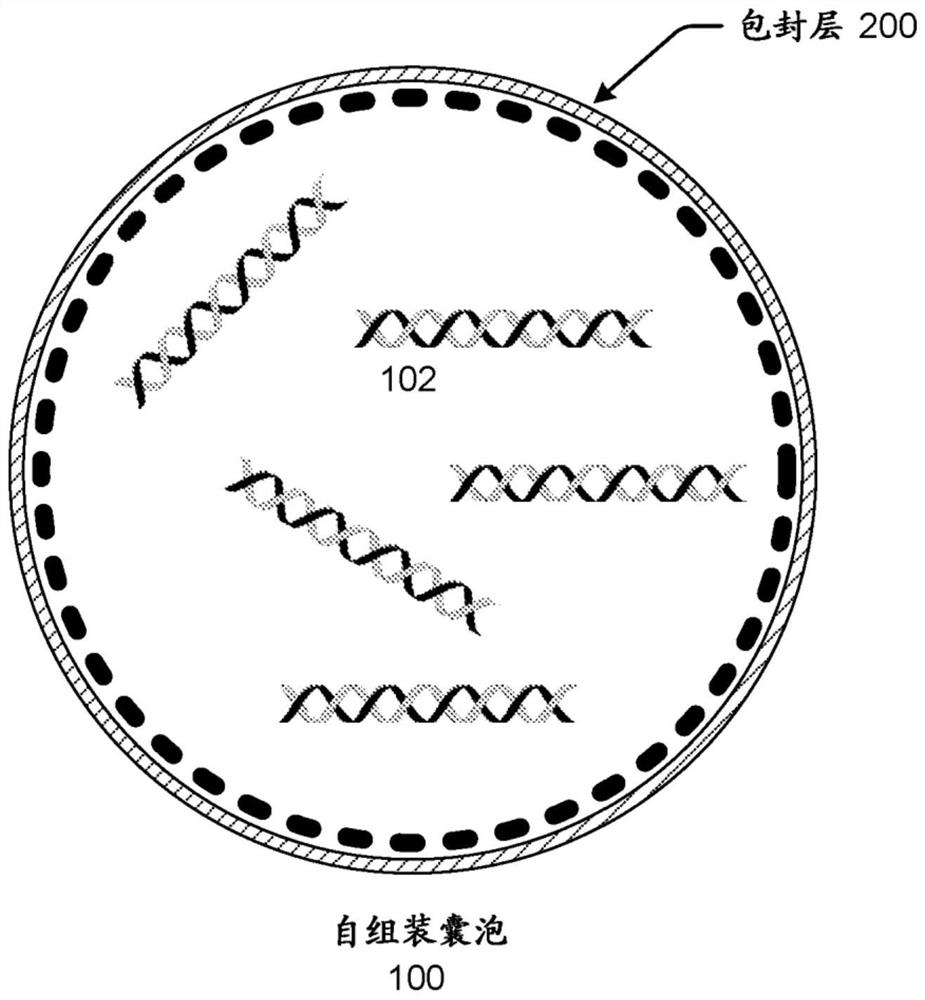
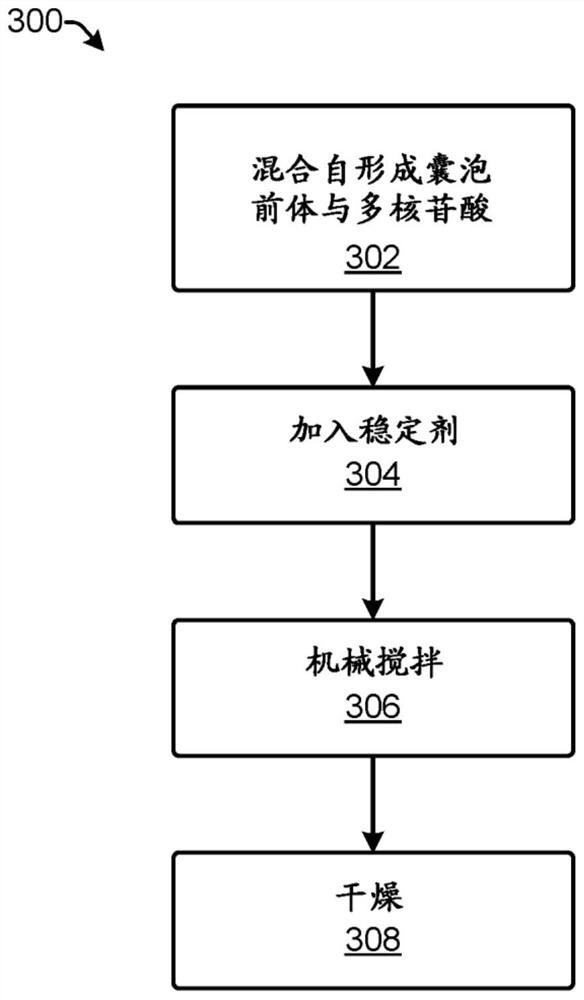
Polynucleotide encapsulation and preservation using self-assembling membranes
Polynucleotides such as DNA are stored inside vesicles formed from self-assembling membranes. The vesicles may be protocells, liposome, micelles, colloidosomes, proteinosomes, or coacervates. The vesicles may include surface functionalization to improve polynucleotide encapsulation and / or to bind polynucleotides having specific sequences. Encapsulation in vesicles provides protection for the polynucleotides. Additional protection is provided by addition of one or more stabilizers. The stabilizer may be nucleic-acid stabilizers that stabilize the polynucleotides or may be a protective structural layer around the vesicles such as a layer of silica. A process for stably storing polynucleotides in vesicles and a process for recovering stored polynucleotides from vesicles are both disclosed. The polynucleotides may be used for storage of digital information.
Owner:MICROSOFT TECH LICENSING LLC
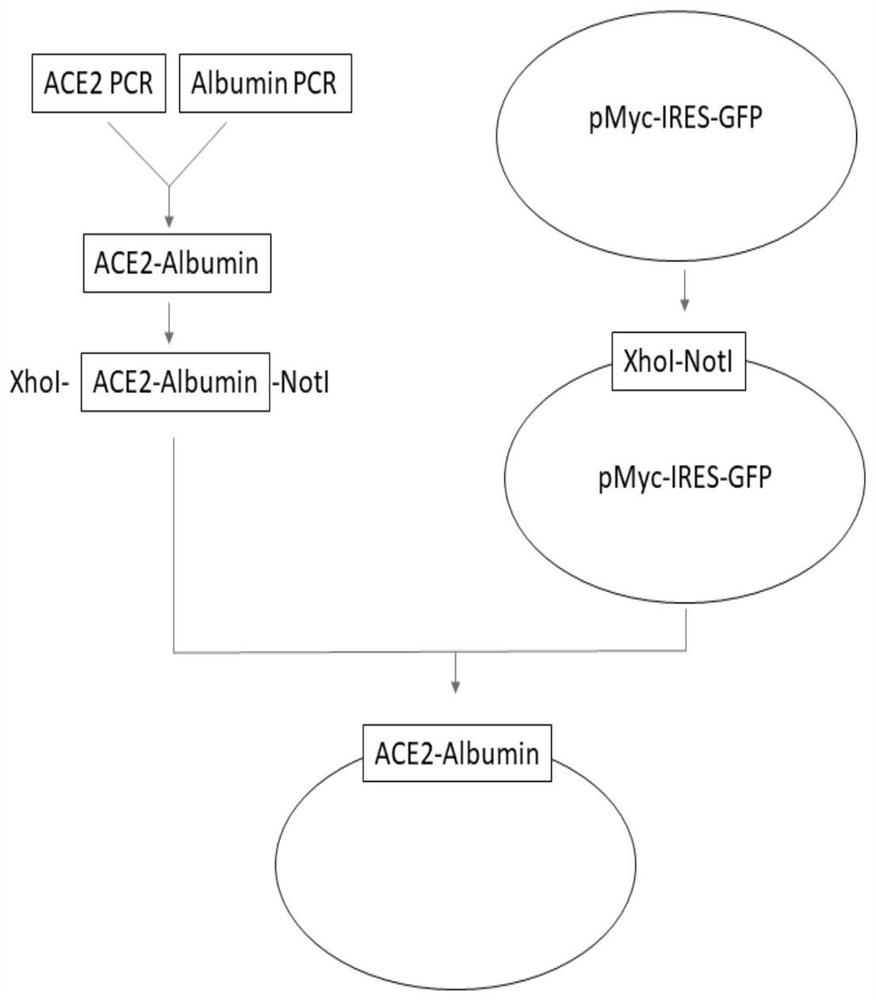
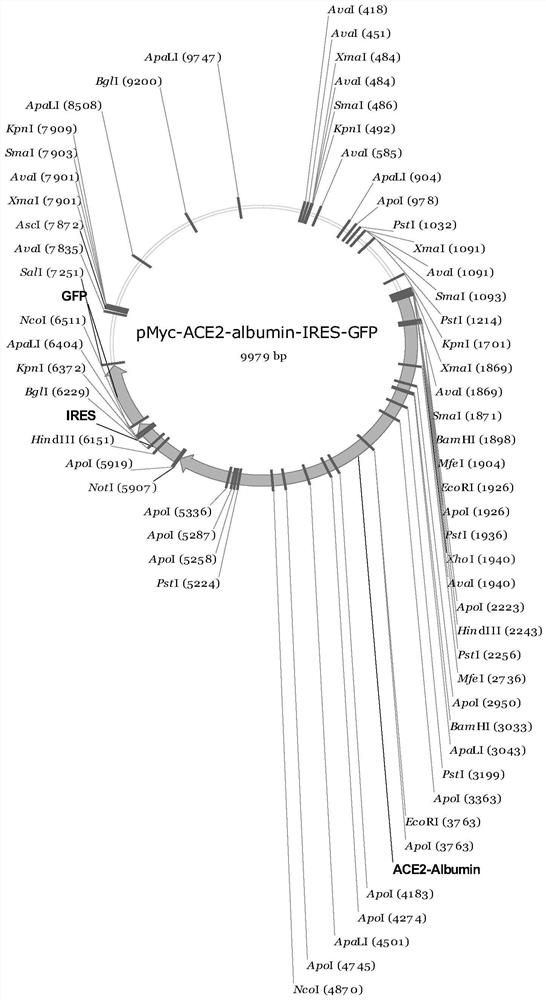
ACE2-Albumin recombinant protein as well as preparation method and application thereof
InactiveCN111635461AImprove stabilityExtended half-lifePeptide/protein ingredientsAntiviralsAlbuminHistone protein
The invention discloses an ACE2-Albumin recombinant protein as well as a preparation method and application thereof. Aiming at the defect of short in-vivo half-life period of existing ACE2 protein, the ACE2 protein and the Albumin protein are subjected to fusion design, so that the in-vivo stability of the ACE2 protein is greatly improved, the half-life period of the ACE2 protein is prolonged, andthe phenomenon of antibody dependence enhancement is avoided. Furthermore, the invention constructs a production vector of the ACE2-Albumin recombinant protein, and provides an efficient method for cloning, screening and transfecting cells. The invention further provides a method for efficiently expressing and purifying the ACE2-Albumin recombinant protein in cells. Preferably, the invention further provides an application method of the ACE2-Albumin recombinant protein in detecting coronaviruses.
Owner:乾元康安(苏州)生物科技有限公司
Hog cholera virus envelope protein oligomeric protein and preparation method and application thereof
ActiveCN110845584AEasy to useGood antigenicitySsRNA viruses positive-senseViral antigen ingredientsClassical swine fever virus CSFVProtein molecules
The invention provides hog cholera virus envelope protein oligomeric protein and a preparation method and application thereof. The oligomeric protein comprises hog cholera virus envelope protein molecules and exogenous oligomerization structural fragments, wherein the hog cholera virus envelope protein molecules are selected from hog cholera virus envelope protein E2 molecules and hog cholera virus envelope protein E0 molecules, the hog cholera virus envelope protein E2 molecules and the exogenous oligomerization structural fragments form the E2 oligomeric protein, and the hog cholera virus envelope protein E0 molecules and the exogenous oligomerization structural fragments form E0 oligomeric protein. The E2 oligomeric protein and the E0 oligomeric protein which serve as the vaccine antigens do not contain any genetic material RNA of hog cholera virus and are safe in production and use. The natural structure and bioactivity of envelope protein in the E2 oligomeric protein and the E0 oligomeric protein are kept, so that the E2 oligomeric protein and the E0 oligomeric protein which serve as the vaccine antigens are evident in immune effect. The oligomeric protein polymerizes the multiple subunit protein of the antigens, overcomes the shortcomings of subunit protein vaccines and is capable of evidently increasing vaccine immunogenicity, high in antigen content and easy in production and purification.
Owner:许雁
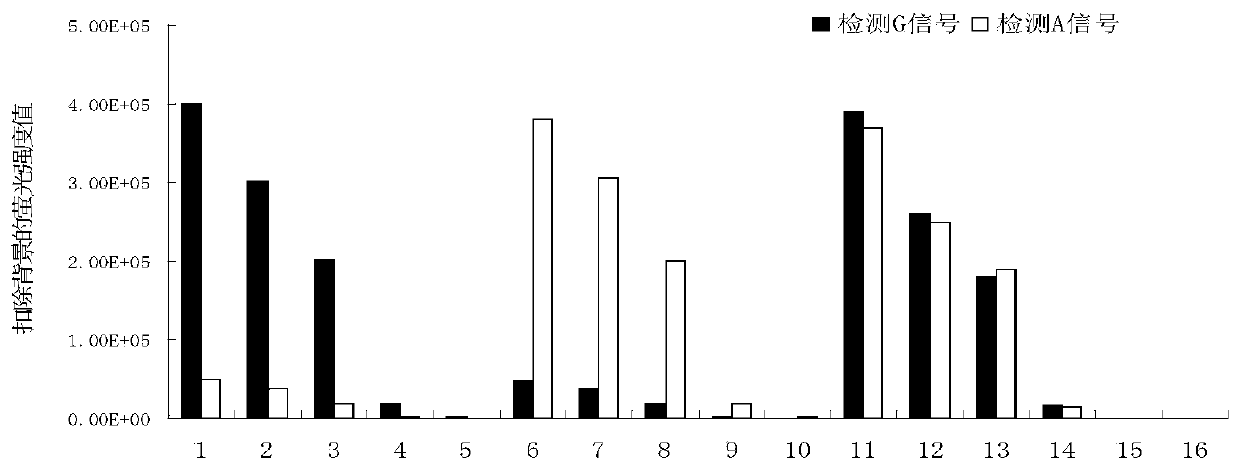
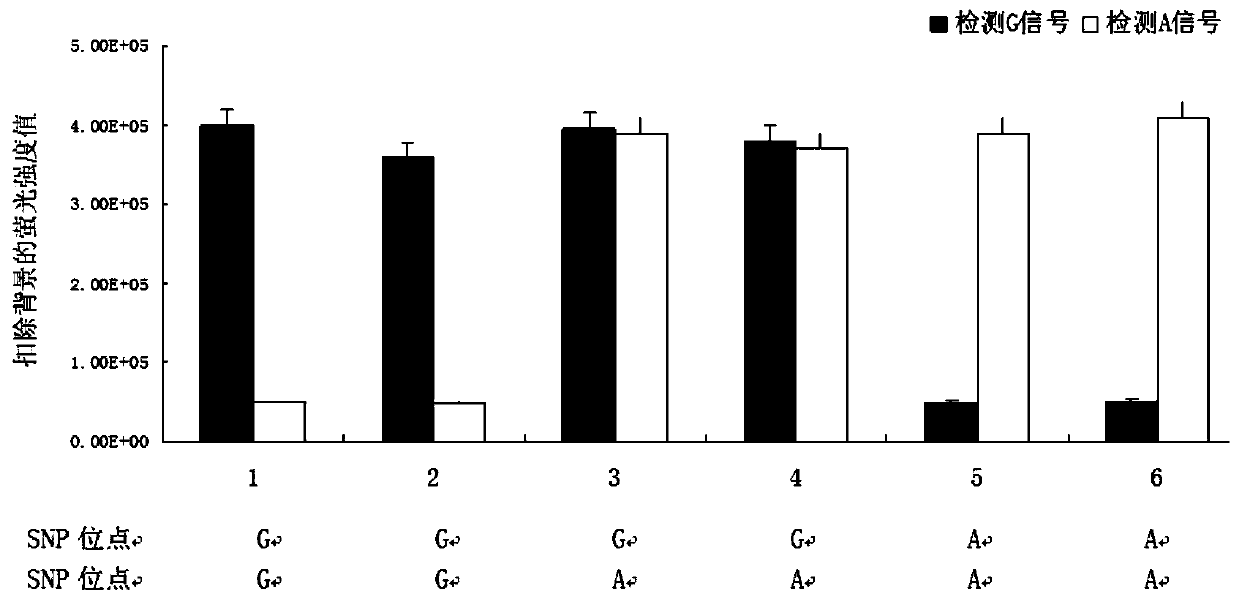
One group of primer for detecting SNP (single nucleotide polymorphism) site rs9263726, crRNA (CRISPR (clustered regularly interspaced short palindromic repeat sequences) RNA) sequence and application of primer
ActiveCN110923314AStrong specificityEasy to judgeMicrobiological testing/measurementAgainst vector-borne diseasesBioinformaticsCRISPR
The invention provides a system for detecting the SNP (single nucleotide polymorphism) site rs9263726 of a HLA-B*5801 gene. The system comprises a primer for detecting the SNP site rs9263726, two (CRISPR (clustered regularly interspaced short palindromic repeat sequences) RNA) sequences, two different CRISPR / cas13 protein systems and reporter probes with different fluorescent marks. When the system disclosed by the invention is used for detecting a mutation type of the SNP site rs9263726 of the HLA-B*5801 gene, the system is convenient in operation, and each detection sample only needs to be carried out in a single tube; a whole reaction process is carried out at a temperature of about 37 DEG C instead of a delicate temperature control element and complex temperature change just like PCR (Polymerase Chain Reaction) amplification, and therefore, the system is suitable for grassroots units to carry out detection by relatively cheap instant detection equipment; and compared with a PCR technology, the system has a higher speed, can obtain an obvious detection signal in 30 min, is high in detection result specificity and can easily judge the type of an allele.
Owner:广州白云山拜迪生物医药有限公司
Recombinant protein body-inducing polypeptides
Polypeptide sequences for inducing recombinant protein bodies are described. The sequences comprise a polyproline II (PPII) structure and / or a proline-rich sequence between two cysteine residues on either end. Recombinant protein bodies are useful for protein production because they allow for simple and efficient purification of high quantities of recombinant protein. In addition, other methods of using recombinant protein bodies, for example, in vaccination and food products, are also described.
Owner:ERA BIOTECH SA
Biological electron microscope sample carbonization method
ActiveCN105021432AChemical physical shrinkage is smallPromote resultsPreparing sample for investigationOsmium tetroxideFine structure
The invention belongs to the field of biological sample preparation, and particularly relates to a biological electron microscope sample carbonization method. Volatile gas of 0.1 M osmium tetroxide liquid is adopted for conducting fumigation and burning on organic biological samples, so that the organic biological samples are completely carbonized to meet the requirement for scanning electron microscope observation. Biological electron microscope samples manufactured through the method have a better expression result and are environmentally friendly and low in carbon, operation is easy and convenient, the chemical and physical contraction rate of all the samples is small, the appearance structure is almost unchanged, and the fine structure is clear. The method breaks through the bottleneck of manufacturing of some electron microscope samples so that some transmission electron microscope samples and scanning electron microscope samples which can not be manufactured in the prior art can be smoothly manufactured and meet the requirement for electron microscope observation. The method can be used for biological electron microscope sample manufacturing of biological sample tissue, bacteria, organic macromolecule protein bodies or organic macromolecule substances.
Owner:FUDAN UNIV
Ecological chain comprehensive treatment method of kitchen waste
InactiveCN110721980APlay a role in enrichmentSite land improvementFeeding-stuffTransportation and packagingEnvironmental engineeringWaste treatment
The invention discloses an ecological chain comprehensive treatment method of kitchen waste, and relates to the field of waste treatment. The method comprises the steps that the kitchen waste is pretreated; the pretreated kitchen waste is digested through multiple kinds of primary organisms; and the kitchen waste and residual kitchen waste obtained after treatment are removed through anaerobic composting. Cockroaches and earthworms exceeding the bearing capacity of the ecological system can be fed to secondary consumers of chickens, toads and the like. Eggs and corpses of chickens and toads can be simply treated, protein bodies can be made, and the method can be used for biological reproduction of carnivorous furbearers.
Owner:NORTH CHINA ELECTRIC POWER UNIV (BAODING)
Encoding gene from anopheles sinensis aquaporin 2 and application of anopheles sinensis aquaporin 2
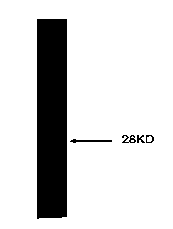
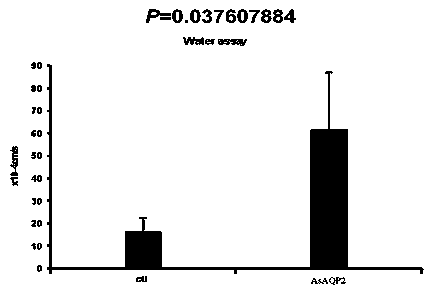
InactiveCN103725689AGood antigenicityInducible productionBacteriaFermentationNucleotideComplementary deoxyribonucleic acid
The invention discloses an encoding gene from anopheles sinensis aquaporin 2 and an application of the anopheles sinensis aquaporin 2, belongs to the technical field of bioengineering, and provides the encoding gene of the anopheles sinensis aquaporin 2, polypeptides synthetized on the basis of the encoding gene, built recombinant protein and an application technology of the anopheles sinensis aquaporin 2 in an insect pesticide. Complementary deoxyribonucleic acid (cDNA) of the anopheles sinensis aquaporin 2 comprises 753 basic groups, has a nucleotide sequence shown in SEQ ID No.1, and is named as (i)AsAQP( / i)2; the anopheles sinensis aquaporin 2 encodes 250 amino acids, has an amino acid sequence shown in SEQ ID No.2, and has 90% of homology with the amino acid sequence (AGAP008842) corresponding to anopheles gambiae. The molecular weight of the anopheles sinensis aquaporin 2 is 26.0kDa; the isoelectric point is 6.40; the anopheles sinensis aquaporin 2 is a stable protein. The in vivo experiment proves that the anopheles sinensis aquaporin 2 has an efficient moisture transport function. 14 amino acids at N-terminal have good antigenicity, and can induce generation of immuno-antibodies. The recombinant protein has a good application prospect in the insect pesticide.
Owner:JIANGSU INST OF PARASITIC DISEASES
Dyeing method for mammal tail tendon medical suture line
The invention relates to a dyeing method for a mammal tail tendon medical suture line. A mammal tail tendon belongs to a collagen composition and pigments can not easily enter proteins to show colors, so the coloring is very difficult; in order to bring pigments into the proteins, the epidermis structures of the proteins need to be damaged by enzymolysis and then the pigments can enter the proteins to show the colors. The dyeing method applies an enzymolysis approach and particularly comprises the steps of firstly extracting the pigments and then carrying out enzymolysis approach for bringing the pigments into the proteins to show the colors. The drying method is the preparation method of the mammal tail tendon medical suture line is finished.
Owner:CHONGZHOU DILONG HAILONG BIOLOGICAL PRODS DEV INST
Double-protein co-emulsification system, preparation method and application thereof
ActiveCN104323235AImprove stabilitySave resourcesCosmetic preparationsPeptide/protein ingredientsChemistryOil separation
The invention discloses a double-protein co-emulsification system, a preparation method and an application thereof. The double-protein co-emulsification system is prepared from 0.5-5 wt% of proteins, and grease and water, wherein a volume ratio of the grease to the water is 2-15:85-98. The preparation method includes following steps: dissolving the proteins in water for enabling the protein to be hydrated fully; and adding the grease with shearing and homogenization. The double-protein co-emulsification system has an excellent stability, is free of phenomena, such as oil separation, layering, precipitation, flocculation and the like, after being stored for a long time, and is free of any bad odor. The double-protein co-emulsification system is free of extra addition of any emulsifier and stabilizing agents and is free of adjustment of pH value of a product. The preparation method is simple, is easy-to-control in technology parameters, is economical and easy to carry out and is suitable for industrialized production. The double-protein co-emulsification system also can be used for preparing enteral nutrition preparations, health-caring foods, medicine preparations, beverages or cosmetics, and especially is suitable for preparing the enteral nutrition preparations.
Owner:SUN YAT SEN UNIV
Method for producing apolipoprotein in plants
InactiveCN103270161ASimplify High DensityApolipeptidesVector-based foreign material introductionBiotechnologyNucleic acid sequencing
Owner:PHILIP MORRIS PROD SA
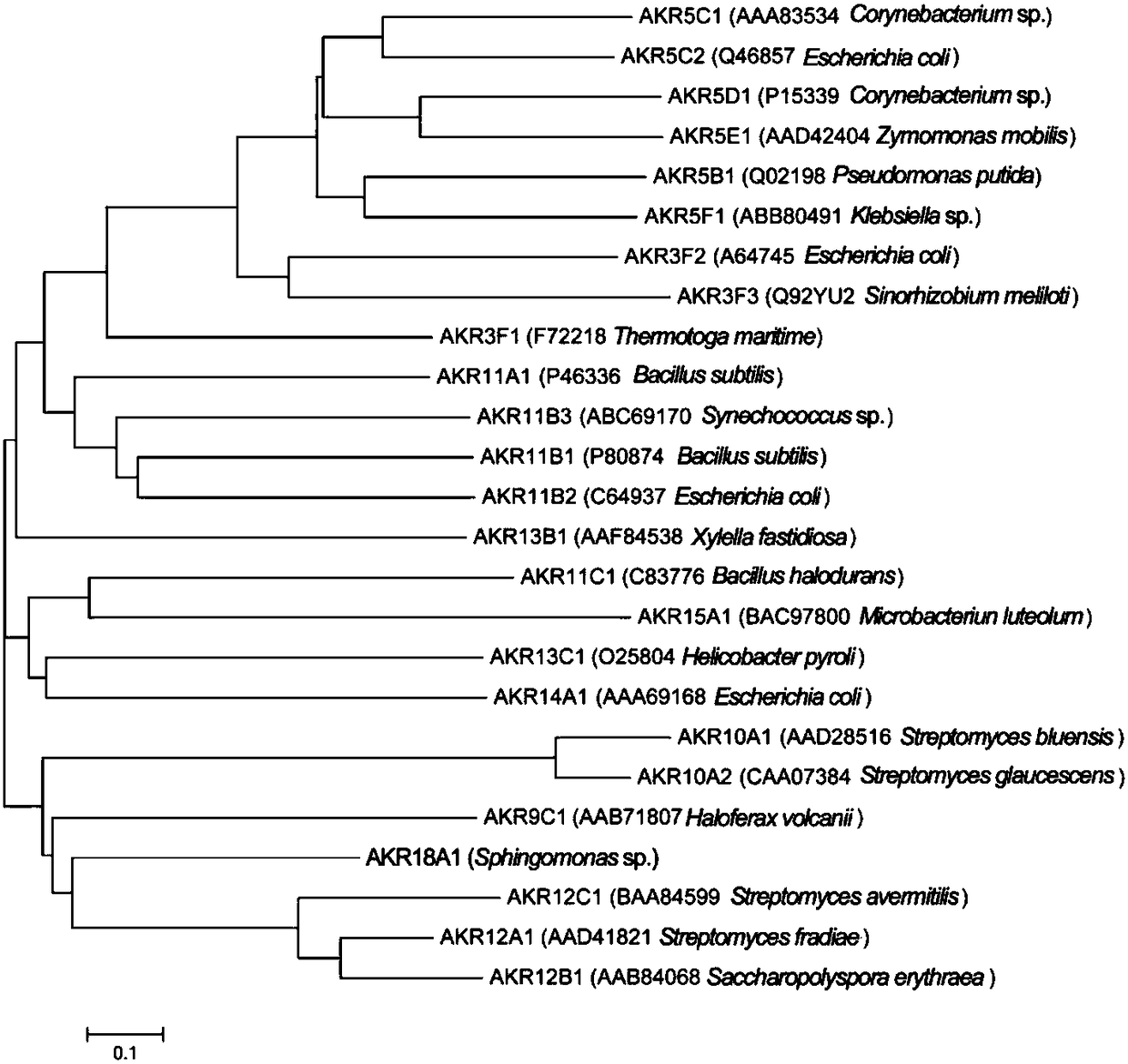
Gene AKR18A1 relevant to detoxification of fusarium toxin and toxic aldehydes and applications of gene AKR18A1
ActiveCN109251933AHigh expressionImprove solubilityAccessory food factorsOxidoreductasesFusarium toxinsAmino acid
The invention discloses a gene AKR18A1 relevant to detoxification of fusarium toxin and toxic aldehyde compounds and applications of the gene AKR18A1. The gene AKR18A1 has the sequence as shown in SEQID NO.1, amino acid coded by the gene AKR18A1 comprises a conservative feature structure element of aldo-keto reductase, and the sequence of the amino acid is as shown in SEQ ID NO.2. Purified protein is obtained through prokaryotic expression of the gene AKR18A1, and the in-vitro experiment proves that the purified protein can oxidize deoxynivalenol, so that 3-keto-deoxynivalenol is formed; andmeanwhile, the protein can act on zearalenone and derivatives of zearalenone, including alpha-zearalenol and beta-zearalenol, and also can effectively degrade the toxic aldehyde compounds, namely, glyoxal and methylglyoxal.
Owner:HUAZHONG AGRI UNIV
Ku70 protein mutant with tumor cell proliferation inhibition function, and gene and application thereof
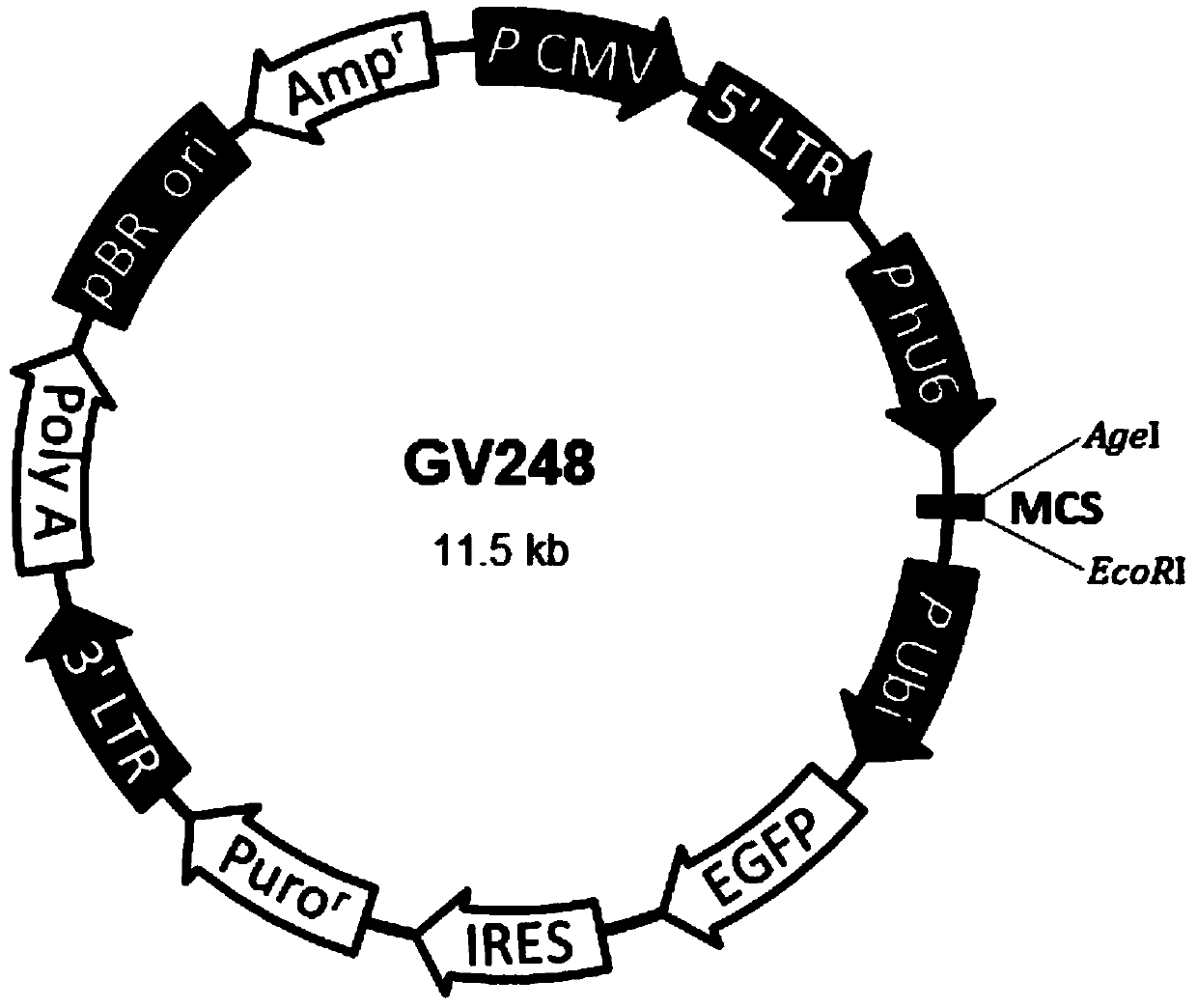
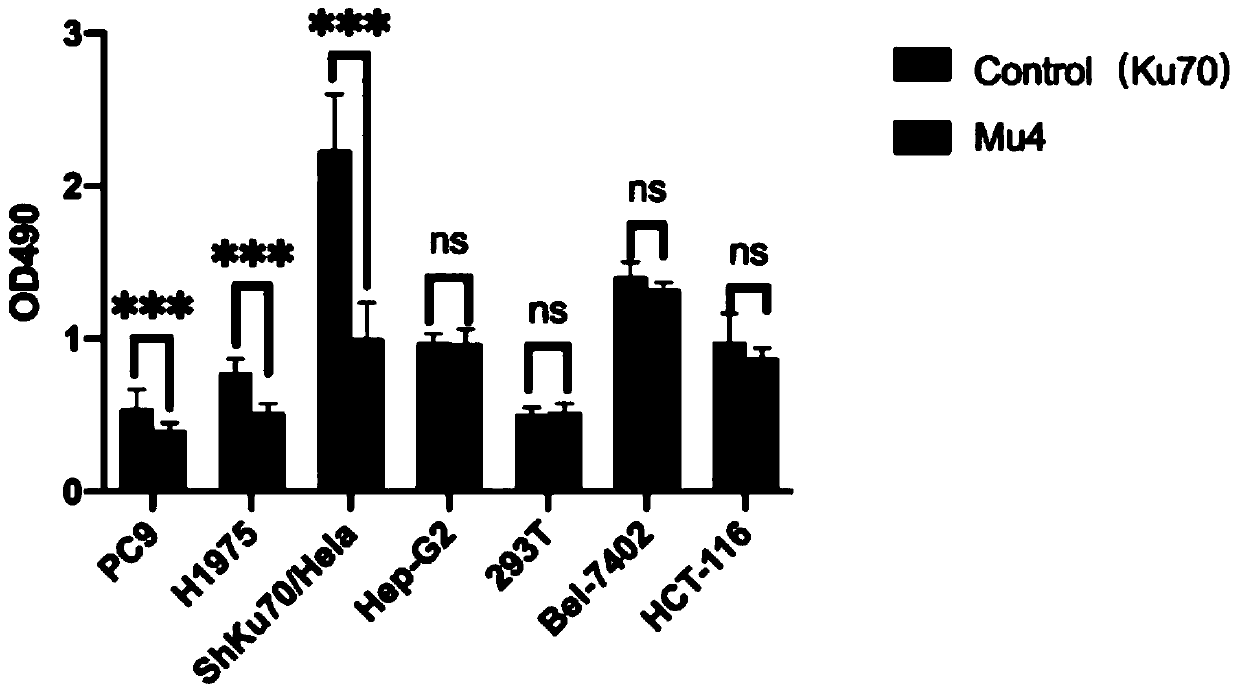
ActiveCN111333712AGood anti-proliferative propertiesPeptide/protein ingredientsFermentationKu70Pharmaceutical drug
The invention discloses a Ku70 protein mutant with a tumor cell proliferation inhibition function, and a gene and application thereof. The amino acid sequence of the Ku70 protein mutant is shown as SEQ ID NO.1. According to the invention, influence of a Ku70 protein on tumor cell proliferation is utilized, and the variant Ku70 protein Mu4 which can effectively inhibit tumor proliferation is constructed through artificial modification. In-vitro anti-tumor activity detection and animal experiments show that the modified protein shows good anti-tumor proliferation characteristics, can be used forresearching the action mechanism of Ku70 participating in tumor cell proliferation, and provides a theoretical basis and model for developing anti-tumor drugs. Therefore, the Ku70 protein mutant hasa certain application prospect in the field of medicines.
Owner:南京华方生物工程有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com